The Pathology Lesion Patterns of Podocytopathies: How and why?
- PMID: 35281116
- PMCID: PMC8907833
- DOI: 10.3389/fcell.2022.838272
The Pathology Lesion Patterns of Podocytopathies: How and why?
Abstract
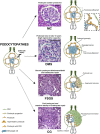
Podocytopathies are a group of proteinuric glomerular disorders driven by primary podocyte injury that are associated with a set of lesion patterns observed on kidney biopsy, i.e., minimal changes, focal segmental glomerulosclerosis, diffuse mesangial sclerosis and collapsing glomerulopathy. These unspecific lesion patterns have long been considered as independent disease entities. By contrast, recent evidence from genetics and experimental studies demonstrated that they represent signs of repeated injury and repair attempts. These ongoing processes depend on the type, length, and severity of podocyte injury, as well as on the ability of parietal epithelial cells to drive repair. In this review, we discuss the main pathology patterns of podocytopathies with a focus on the cellular and molecular response of podocytes and parietal epithelial cells.
Keywords: collapsing glomerulopathy; diffuse mesangial sclerosis; focal segmental glomerulosclerosis; minimal change; minimal change disease; parietal epithelial cells; podocytopathies; renal progenitor.
Copyright © 2022 Ravaglia, Melica, Angelotti, De Chiara, Romagnani and Lasagni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abid Q., Best Rocha A., Larsen C. P., Schulert G., Marsh R., Yasin S., et al. (2020). APOL1-Associated Collapsing Focal Segmental Glomerulosclerosis in a Patient with Stimulator of Interferon Genes (STING)-Associated Vasculopathy with Onset in Infancy (SAVI). Am. J. Kidney Dis. 75, 287–290. 10.1053/j.ajkd.2019.07.010 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

