Three-Dimensional Model of Sub-Plasmalemmal Ca2+ Microdomains Evoked by T Cell Receptor/CD3 Complex Stimulation
- PMID: 35281279
- PMCID: PMC8906516
- DOI: 10.3389/fmolb.2022.811145
Three-Dimensional Model of Sub-Plasmalemmal Ca2+ Microdomains Evoked by T Cell Receptor/CD3 Complex Stimulation
Abstract
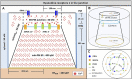
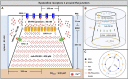
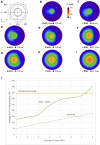
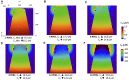
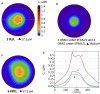
Ca2+ signalling plays an essential role in T cell activation, which is a key step to start an adaptive immune response. During the transition from a quiescent to a fully activated state, Ca2+ microdomains of reduced spatial and temporal extents develop in the junctions between the plasma membrane and the endoplasmic reticulum (ER). These microdomains rely on Ca2+ entry from the extracellular medium, via the ORAI1/STIM1/STIM2 system that mediates store operated Ca2+ entry Store operated calcium entry. The mechanism leading to local store depletion and subsequent Ca2+ entry depends on the activation state of the cells. The initial, smaller microdomains are triggered by D-myo-inositol 1,4,5-trisphosphate (IP3) signalling in response to T cell adhesion. T cell receptor (TCR)/CD3 stimulation then initiates nicotinic acid adenine dinucleotide phosphate signalling, which activates ryanodine receptors (RYR). We have recently developed a mathematical model to elucidate the spatiotemporal Ca2+ dynamics of the microdomains triggered by IP3 signalling in response to T cell adhesion (Gil et al., 2021). This reaction-diffusion model describes the evolution of the cytosolic and endoplasmic reticulum Ca2+ concentrations in a three-dimensional ER-PM junction and was solved using COMSOL Multiphysics. Modelling predicted that adhesion-dependent microdomains result from the concerted activity of IP3 receptors and pre-formed ORAI1-STIM2 complexes. In the present study, we extend this model to include the role of RYRs rapidly after TCR/CD3 stimulation. The involvement of STIM1, which has a lower KD for Ca2+ than STIM2, is also considered. Detailed 3D spatio-temporal simulations show that these Ca2+ microdomains rely on the concerted opening of ∼7 RYRs that are simultaneously active in response to the increase in NAADP induced by T cell stimulation. Opening of these RYRs provoke a local depletion of ER Ca2+ that triggers Ca2+ flux through the ORAI1 channels. Simulations predict that RYRs are most probably located around the junction and that the increase in junctional Ca2+ concentration results from the combination between diffusion of Ca2+ released through the RYRs and Ca2+ entry through ORAI1 in the junction. The computational model moreover provides a tool allowing to investigate how Ca2+ microdomains occur, extend and interact in various states of T cell activation.
Keywords: COMSOL; ER-PM junctions; NAADP; T cells; ca2+ signalling; computational model; ryanodine receptors; store operated calcium entry.
Copyright © 2022 Gil, Diercks, Guse and Dupont.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Ahmad M., Ong H. L., Saadi H., Son G. Y., Shokatian Z., Terry L. E., et al. (2021). Functional Communication between IP3R and STIM2 at Sub-threshold Stimuli Is a Critical Checkpoint for Initiation of SOCE. Proc. Natl. Acad. Sci. U S A 119 (3), e2114928118. 10.1073/pnas.2114928118 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

