Raman spectroscopic insight into osteoarthritic cartilage regeneration by mRNA therapeutics encoding cartilage-anabolic transcription factor Runx1
- PMID: 35281370
- PMCID: PMC8913780
- DOI: 10.1016/j.mtbio.2022.100210
Raman spectroscopic insight into osteoarthritic cartilage regeneration by mRNA therapeutics encoding cartilage-anabolic transcription factor Runx1
Abstract
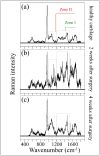
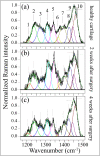
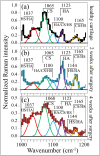
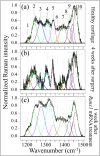
While joint arthroplasty remains nowadays the most popular option available to repair chronically degenerated osteoarthritic joints, possibilities are recently emerging for regeneration of damaged cartilage rather than its replacement with artificial biomaterials. This latter strategy could allow avoiding the quite intrusive surgical procedures associated with total joint replacement. Building upon this notion, we first apply Raman spectroscopy to characterize diseased cartilage in a mice model of instability-induced knee osteoarthritis (OA) upon medial collateral ligament (MCL) and medial meniscus (MM) transections. Then, we examine the same OA model after cartilage regeneration by means of messenger RNA (mRNA) delivery of a cartilage-anabolic runt-related transcription factor 1 (RUNX1). Raman spectroscopy is shown to substantiate at the molecular scale the therapeutic effect of the Runx1 mRNA cartilage regeneration approach. This study demonstrates how the Raman spectroscopic method could support and accelerate the development of new therapies for cartilage diseases.
Keywords: Cartilage regeneration; Cartilage-anabolic transcription factor; Raman spectroscopy; mRNA therapeutics.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment.Sci Rep. 2016 Jan 5;6:18743. doi: 10.1038/srep18743. Sci Rep. 2016. PMID: 26728350 Free PMC article.
-
Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability.Arthritis Rheum. 2006 Aug;54(8):2462-70. doi: 10.1002/art.22041. Arthritis Rheum. 2006. PMID: 16868966
-
Runx1 Activities in Superficial Zone Chondrocytes, Osteoarthritic Chondrocyte Clones and Response to Mechanical Loading.J Cell Physiol. 2015 Feb;230(2):440-8. doi: 10.1002/jcp.24727. J Cell Physiol. 2015. PMID: 25078095 Free PMC article.
-
A Comprehensive Review of Stem Cells for Cartilage Regeneration in Osteoarthritis.Adv Exp Med Biol. 2018;1089:23-36. doi: 10.1007/5584_2018_205. Adv Exp Med Biol. 2018. PMID: 29725971 Review.
-
Cartilage regeneration for treatment of osteoarthritis: a paradigm for nonsurgical intervention.Ther Adv Musculoskelet Dis. 2015 Jun;7(3):76-87. doi: 10.1177/1759720X15576866. Ther Adv Musculoskelet Dis. 2015. PMID: 26029269 Free PMC article. Review.
Cited by
-
Three-Dimensional Culture of Cartilage Tissue on Nanogel-Cross-Linked Porous Freeze-Dried Gel Scaffold for Regenerative Cartilage Therapy: A Vibrational Spectroscopy Evaluation.Int J Mol Sci. 2022 Jul 22;23(15):8099. doi: 10.3390/ijms23158099. Int J Mol Sci. 2022. PMID: 35897669 Free PMC article.
-
Osteoarthritis: Mechanisms and Therapeutic Advances.MedComm (2020). 2025 Aug 1;6(8):e70290. doi: 10.1002/mco2.70290. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40757100 Free PMC article. Review.
-
Spectroscopic Analysis of the Extracellular Matrix in Naked Mole-Rat Temporomandibular Joints.Gels. 2025 May 30;11(6):414. doi: 10.3390/gels11060414. Gels. 2025. PMID: 40558714 Free PMC article.
-
Blood-Induced Arthropathy: A Major Disabling Complication of Haemophilia.J Clin Med. 2023 Dec 30;13(1):225. doi: 10.3390/jcm13010225. J Clin Med. 2023. PMID: 38202232 Free PMC article. Review.
-
Current Trends in In Vitro Diagnostics Using Surface-Enhanced Raman Scattering in Translational Biomedical Research.Biosensors (Basel). 2025 Apr 22;15(5):265. doi: 10.3390/bios15050265. Biosensors (Basel). 2025. PMID: 40422004 Free PMC article. Review.
References
-
- Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2001;10:432–463. - PubMed
-
- Ye K., Di Bella C., Myers D.E., Choong P.F.M. The osteochondral dilemma, review of current management and future trends. ANZ J. Surg. 2014;84:211–217. - PubMed
-
- Hawker G.A., Stanaitis I. Osteoarthritis year in review 2014, clinical. Osteoarthritis Cartilage. 2014;22:1953–1957. - PubMed
-
- Kaul G., Cucchiarini M., Remberger K., Kohn D., Madry H. Failed cartilage repair for early osteoarthritis defect, a biochemical, histological and immunohystochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg. Sports Traumatol. Arthrosc. 2012;20:2315–2324. - PubMed
-
- Felson D.T., Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343–1355. - PubMed
LinkOut - more resources
Full Text Sources

