Advanced Imaging Techniques for Newly Diagnosed and Recurrent Gliomas
- PMID: 35281485
- PMCID: PMC8904563
- DOI: 10.3389/fnins.2022.787755
Advanced Imaging Techniques for Newly Diagnosed and Recurrent Gliomas
Abstract
Management of gliomas following initial diagnosis requires thoughtful presurgical planning followed by regular imaging to monitor treatment response and survey for new tumor growth. Traditional MR imaging modalities such as T1 post-contrast and T2-weighted sequences have long been a staple of tumor diagnosis, surgical planning, and post-treatment surveillance. While these sequences remain integral in the management of gliomas, advances in imaging techniques have allowed for a more detailed characterization of tumor characteristics. Advanced MR sequences such as perfusion, diffusion, and susceptibility weighted imaging, as well as PET scans have emerged as valuable tools to inform clinical decision making and provide a non-invasive way to help distinguish between tumor recurrence and pseudoprogression. Furthermore, these advances in imaging have extended to the operating room and assist in making surgical resections safer. Nevertheless, surgery, chemotherapy, and radiation treatment continue to make the interpretation of MR changes difficult for glioma patients. As analytics and machine learning techniques improve, radiomics offers the potential to be more quantitative and personalized in the interpretation of imaging data for gliomas. In this review, we describe the role of these newer imaging modalities during the different stages of management for patients with gliomas, focusing on the pre-operative, post-operative, and surveillance periods. Finally, we discuss radiomics as a means of promoting personalized patient care in the future.
Keywords: PET scanning; glioma; imaging; progression; pseudoprogression; radiomics; recurrence.
Copyright © 2022 Carrete, Young and Cha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


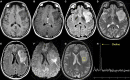
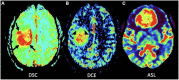
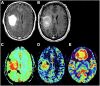
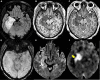
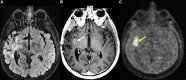
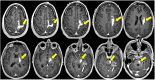

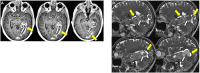

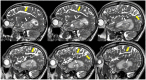

References
-
- Abel R., Jones J., Mandelin P., Cen S., Pagnini P. (2012). Distinguishing pseudoprogression from true progression by FLAIR volumetric characteristics compared to 45 Gy isodose volumes in treated glioblastoma patients. Int. J. Radiat. Oncol. 84:S275. 10.1016/j.ijrobp.2012.07.716 - DOI
-
- Albert N. L., Weller M., Suchorska B., Galldiks N., Soffietti R., Kim M. M., et al. (2016). Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 18 1199–1208. 10.1093/neuonc/now058 - DOI - PMC - PubMed
-
- Barajas R. F., Phillips J. J., Parvataneni R., Molinaro A., Essock-Burns E., Bourne G., et al. (2012). Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro-Oncology 14 942–954. 10.1093/neuonc/nos128 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

