The BrainScaleS-2 Accelerated Neuromorphic System With Hybrid Plasticity
- PMID: 35281488
- PMCID: PMC8907969
- DOI: 10.3389/fnins.2022.795876
The BrainScaleS-2 Accelerated Neuromorphic System With Hybrid Plasticity
Abstract
Since the beginning of information processing by electronic components, the nervous system has served as a metaphor for the organization of computational primitives. Brain-inspired computing today encompasses a class of approaches ranging from using novel nano-devices for computation to research into large-scale neuromorphic architectures, such as TrueNorth, SpiNNaker, BrainScaleS, Tianjic, and Loihi. While implementation details differ, spiking neural networks-sometimes referred to as the third generation of neural networks-are the common abstraction used to model computation with such systems. Here we describe the second generation of the BrainScaleS neuromorphic architecture, emphasizing applications enabled by this architecture. It combines a custom analog accelerator core supporting the accelerated physical emulation of bio-inspired spiking neural network primitives with a tightly coupled digital processor and a digital event-routing network.
Keywords: neuromorphic computing; neuroscientific modeling; physical modeling; plasticity; spiking neural network accelerator.
Copyright © 2022 Pehle, Billaudelle, Cramer, Kaiser, Schreiber, Stradmann, Weis, Leibfried, Müller and Schemmel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
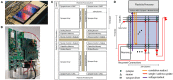
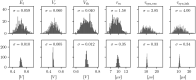
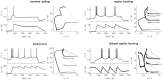



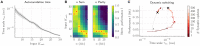


References
-
- Aamir S. A., Stradmann Y., Müller P., Pehle C., Hartel A., Grübl A., et al. . (2018b). An accelerated LIF neuronal network array for a large-scale mixed-signal neuromorphic architecture. IEEE Trans. Circ. Syst. 65, 4299–4312. 10.1109/TCSI.2018.2840718 - DOI
-
- Barton P. I., Lee C. K. (2002). Modeling, simulation, sensitivity analysis, and optimization of hybrid systems. ACM Trans. Model. Comput. Simul. 12, 256–289. 10.1145/643120.643122 - DOI
-
- Bellec G., Salaj D., Subramoney A., Legenstein R., Maass W. (2018). Long short-term memory and learning-to-learn in networks of spiking neurons. arXiv preprint arXiv:1803.09574.
LinkOut - more resources
Full Text Sources

