Anti-inflammatory α-Melanocyte-Stimulating Hormone Protects Retina After Ischemia/Reperfusion Injury in Type I Diabetes
- PMID: 35281489
- PMCID: PMC8914517
- DOI: 10.3389/fnins.2022.799739
Anti-inflammatory α-Melanocyte-Stimulating Hormone Protects Retina After Ischemia/Reperfusion Injury in Type I Diabetes
Abstract
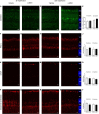
Retinal ischemia/reperfusion (I/R) injury is a major cause of vision loss in many ocular diseases. Retinal I/R injury is common in diabetic retinopathy, which as a result of hyperglycemia damages the retina and can cause blindness if left untreated. Inflammation is a major contributing factor in the pathogenesis of I/R injury. α-Melanocyte-stimulating hormone (α-MSH) is an anti-inflammatory peptide hormone that has displayed protective effects against I/R-induced organ damages. Here, we aimed to investigate the protective role of α-MSH on I/R-induced diabetic retinal damage using hyperglycemic C57BL/6J Ins2Akita/+ mice. Experimental I/R injury was induced by blocking the right middle cerebral artery (MCA) for 2 h followed by 2 h or 22 h of reperfusion using the intraluminal method. Since ophthalmic artery originates proximal to the origin of the MCA, the filament also blocked blood supply to the retina. Upon treatment with α-MSH at 1 h after ischemia and 1 h after reperfusion, animals displayed significant improvement in amplitudes of b-wave and oscillatory potentials during electroretinography. α-MSH also prevented I/R-induced histological alterations and inhibited the development of retinal swelling. Loss of retinal ganglion cells as well as oxidative stress were significantly attenuated in the α-MSH-treated retinae. Level of interleukin 10 was significantly increased after α-MSH treatment. Moreover, gene expression of glutamate aspartate transporter 1, monocarboxylate transporter (MCT) 1 and MCT-2 were significantly higher after α-MSH administration. In conclusion, α-MSH mitigates the severity of I/R-induced retinal damage under hyperglycemic condition. These beneficial effects of α-MSH may have important therapeutic implications against retinal I/R injury under hyperglycemic condition.
Keywords: diabetes; inflammation; ischemia; oxidative stress; reperfusion; retina.
Copyright © 2022 Goit, Taylor and Lo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





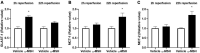
Similar articles
-
Neuropeptide α-Melanocyte-Stimulating Hormone Promotes Neurological Recovery and Repairs Cerebral Ischemia/Reperfusion Injury in Type 1 Diabetes.Neurochem Res. 2022 Feb;47(2):394-408. doi: 10.1007/s11064-021-03453-4. Epub 2021 Sep 29. Neurochem Res. 2022. PMID: 34586586
-
Α-Melanocyte-Stimulating Hormone Protects Early Diabetic Retina from Blood-Retinal Barrier Breakdown and Vascular Leakage via MC4R.Cell Physiol Biochem. 2018;45(2):505-522. doi: 10.1159/000487029. Epub 2018 Jan 25. Cell Physiol Biochem. 2018. PMID: 29402864
-
Protective effect of alpha-melanocyte-stimulating hormone (α-MSH) on the recovery of ischemia/reperfusion (I/R)-induced retinal damage in a rat model.J Mol Neurosci. 2013 Jul;50(3):558-70. doi: 10.1007/s12031-013-9998-3. Epub 2013 Mar 17. J Mol Neurosci. 2013. PMID: 23504281 Free PMC article.
-
alpha-Melanocyte-stimulating hormone and acute renal failure.Curr Opin Nephrol Hypertens. 1998 Jul;7(4):413-7. doi: 10.1097/00041552-199807000-00011. Curr Opin Nephrol Hypertens. 1998. PMID: 9690041 Review.
-
[In vivo evaluation of leukocyte dynamics in the retinal and choroidal circulation].Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):910-22. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643293 Review. Japanese.
Cited by
-
Immunological Landscape of Retinal Ischemia-Reperfusion Injury: Insights into Resident and Peripheral Immune Cell Responses.Aging Dis. 2024 Mar 1;16(1):115-36. doi: 10.14336/AD.2024.0129. Online ahead of print. Aging Dis. 2024. PMID: 38502592 Free PMC article. Review.
-
The Therapeutic Effects of Purified Cortrophin Gel on Experimental Autoimmune Uveitis.Ocul Immunol Inflamm. 2025 Jul 22:1-9. doi: 10.1080/09273948.2025.2532821. Online ahead of print. Ocul Immunol Inflamm. 2025. PMID: 40694652 Free PMC article.
-
Alpha-Melanocyte-Stimulating Hormone Maintains Retinal Homeostasis after Ischemia/Reperfusion.Biomolecules. 2024 Apr 27;14(5):525. doi: 10.3390/biom14050525. Biomolecules. 2024. PMID: 38785932 Free PMC article.
-
Stimulating the Melanocortin System in Uveitis and Diabetes Preserves the Structure and Anti-Inflammatory Activity of the Retina.Int J Mol Sci. 2023 Apr 8;24(8):6928. doi: 10.3390/ijms24086928. Int J Mol Sci. 2023. PMID: 37108092 Free PMC article.
-
Key immune regulators in retinal ischemia-reperfusion injury via RNA sequencing.Int J Ophthalmol. 2025 Jul 18;18(7):1237-1251. doi: 10.18240/ijo.2025.07.06. eCollection 2025. Int J Ophthalmol. 2025. PMID: 40688775 Free PMC article.
References
LinkOut - more resources
Full Text Sources

