Mechanistic Insights Into Gut Microbiome Dysbiosis-Mediated Neuroimmune Dysregulation and Protein Misfolding and Clearance in the Pathogenesis of Chronic Neurodegenerative Disorders
- PMID: 35281490
- PMCID: PMC8914070
- DOI: 10.3389/fnins.2022.836605
Mechanistic Insights Into Gut Microbiome Dysbiosis-Mediated Neuroimmune Dysregulation and Protein Misfolding and Clearance in the Pathogenesis of Chronic Neurodegenerative Disorders
Abstract
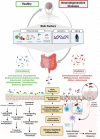
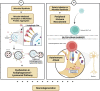
The human gut microbiota is a complex, dynamic, and highly diverse community of microorganisms. Beginning as early as in utero fetal development and continuing through birth to late-stage adulthood, the crosstalk between the gut microbiome and brain is essential for modulating various metabolic, neurodevelopmental, and immune-related pathways. Conversely, microbial dysbiosis - defined as alterations in richness and relative abundances - of the gut is implicated in the pathogenesis of several chronic neurological and neurodegenerative disorders. Evidence from large-population cohort studies suggests that individuals with neurodegenerative conditions have an altered gut microbial composition as well as microbial and serum metabolomic profiles distinct from those in the healthy population. Dysbiosis is also linked to psychiatric and gastrointestinal complications - comorbidities often associated with the prodromal phase of Parkinson's disease (PD) and Alzheimer's disease (AD). Studies have identified potential mediators that link gut dysbiosis and neurological disorders. Recent findings have also elucidated the potential mechanisms of disease pathology in the enteric nervous system prior to the onset of neurodegeneration. This review highlights the functional pathways and mechanisms, particularly gut microbe-induced chronic inflammation, protein misfolding, propagation of disease-specific pathology, defective protein clearance, and autoimmune dysregulation, linking gut microbial dysbiosis and neurodegeneration. In addition, we also discuss how pathogenic transformation of microbial composition leads to increased endotoxin production and fewer beneficial metabolites, both of which could trigger immune cell activation and enteric neuronal dysfunction. These can further disrupt intestinal barrier permeability, aggravate the systemic pro-inflammatory state, impair blood-brain barrier permeability and recruit immune mediators leading to neuroinflammation and neurodegeneration. Continued biomedical advances in understanding the microbiota-gut-brain axis will extend the frontier of neurodegenerative disorders and enable the utilization of novel diagnostic and therapeutic strategies to mitigate the pathological burden of these diseases.
Keywords: Alzheimer’s and Parkinson’s diseases; gut inflammation; gut metabolome; gut microbiota; microbiome; neurodegeneration; neuroinflammation; protein aggregation.
Copyright © 2022 Padhi, Worth, Zenitsky, Jin, Sambamurti, Anantharam, Kanthasamy and Kanthasamy.
Conflict of interest statement
AGK has an equity interest in PK Biosciences Corporation and Probiome Therapeutics located in Ames, IA, United States. The terms of this arrangement have been reviewed and approved by Iowa State University and University of Georgia in accordance with its conflict-of-interest policies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle.Pharmacol Ther. 2022 Mar;231:107988. doi: 10.1016/j.pharmthera.2021.107988. Epub 2021 Sep 16. Pharmacol Ther. 2022. PMID: 34536490 Review.
-
Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer's disease.Life Sci. 2021 Jan 1;264:118627. doi: 10.1016/j.lfs.2020.118627. Epub 2020 Oct 22. Life Sci. 2021. PMID: 33169684 Review.
-
Gut microbiota defined epigenomes of Alzheimer's and Parkinson's diseases reveal novel targets for therapy.Epigenomics. 2024 Jan;16(1):57-77. doi: 10.2217/epi-2023-0342. Epub 2023 Dec 13. Epigenomics. 2024. PMID: 38088063 Free PMC article. Review.
-
Impact of Microbiome-Brain Communication on Neuroinflammation and Neurodegeneration.Int J Mol Sci. 2023 Oct 5;24(19):14925. doi: 10.3390/ijms241914925. Int J Mol Sci. 2023. PMID: 37834373 Free PMC article. Review.
-
Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: consequences for neurodegeneration.Front Cell Infect Microbiol. 2024 Feb 16;14:1348279. doi: 10.3389/fcimb.2024.1348279. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38435303 Free PMC article. Review.
Cited by
-
Molecular and cellular mechanisms leading to catatonia: an integrative approach from clinical and preclinical evidence.Front Mol Neurosci. 2022 Sep 29;15:993671. doi: 10.3389/fnmol.2022.993671. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36245923 Free PMC article. Review.
-
Gut microbiota and Parkinson's disease: potential links and the role of fecal microbiota transplantation.Front Aging Neurosci. 2024 Nov 29;16:1479343. doi: 10.3389/fnagi.2024.1479343. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39679259 Free PMC article. Review.
-
Identification of the Intestinal Microbes Associated with Locomotion.Int J Mol Sci. 2023 Jul 13;24(14):11392. doi: 10.3390/ijms241411392. Int J Mol Sci. 2023. PMID: 37511151 Free PMC article.
-
Identification of Muscle Strength-Related Gut Microbes through Human Fecal Microbiome Transplantation.Int J Mol Sci. 2024 Jan 4;25(1):662. doi: 10.3390/ijms25010662. Int J Mol Sci. 2024. PMID: 38203833 Free PMC article.
-
Resolution of Chronic Inflammation, Restoration of Epigenetic Disturbances and Correction of Dysbiosis as an Adjunctive Approach to the Treatment of Atopic Dermatitis.Cells. 2024 Nov 18;13(22):1899. doi: 10.3390/cells13221899. Cells. 2024. PMID: 39594647 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

