Frontotemporal Dementia and Glucose Metabolism
- PMID: 35281504
- PMCID: PMC8906510
- DOI: 10.3389/fnins.2022.812222
Frontotemporal Dementia and Glucose Metabolism
Abstract
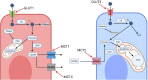
Frontotemporal dementia (FTD), hallmarked by antero-temporal degeneration in the human brain, is the second most common early onset dementia. FTD is a diverse disease with three main clinical presentations, four different identified proteinopathies and many disease-associated genes. The exact pathophysiology of FTD remains to be elucidated. One common characteristic all forms of FTD share is the dysregulation of glucose metabolism in patients' brains. The brain consumes around 20% of the body's energy supply and predominantly utilizes glucose as a fuel. Glucose metabolism dysregulation could therefore be extremely detrimental for neuronal health. Research into the association between glucose metabolism and dementias has recently gained interest in Alzheimer's disease. FTD also presents with glucose metabolism dysregulation, however, this remains largely an unexplored area. A better understanding of the link between FTD and glucose metabolism may yield further insight into FTD pathophysiology and aid the development of novel therapeutics. Here we review our current understanding of FTD and glucose metabolism in the brain and discuss the evidence of impaired glucose metabolism in FTD. Lastly, we review research potentially suggesting a causal relationship between FTD proteinopathies and impaired glucose metabolism in FTD.
Keywords: C9orf72; C9orf72 ALS/FTD; FTD; FUS; MAPT; TDP-42; glucose.
Copyright © 2022 Garrett and Niccoli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aldana B. I., Zhang Y., Jensen P., Chandrasekaran A., Christensen S. K., Nielsen T. T., et al. (2020). Glutamate-glutamine homeostasis is perturbed in neurons and astrocytes derived from patient iPSC models of frontotemporal dementia. Mol. Brain 13:125. 10.1186/s13041-020-00658-6 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

