Ovulation suppression following subcutaneous administration of depot medroxyprogesterone acetate
- PMID: 35281554
- PMCID: PMC8907671
- DOI: 10.1016/j.conx.2022.100073
Ovulation suppression following subcutaneous administration of depot medroxyprogesterone acetate
Abstract
Objectives: To characterize the relationship between serum medroxyprogesterone acetate (MPA) concentrations and ovulation suppression, and to estimate the risk of ovulation for investigational subcutaneous regimens of Depo-Provera CI (Depo-Provera) and Depo-subQ Provera 104 (Depo-subQ).
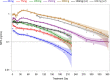
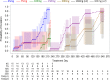
Study design: We performed a secondary analysis of 2 studies that assessed the pharmacokinetics and pharmacodynamics of MPA when Depo-Provera is administered subcutaneously rather than by the labeled intramuscular route. Each woman received a single 45 mg to 300 mg subcutaneous injection of Depo-Provera, a single 104 mg subcutaneous injection of Depo-subQ, or 2 injections of Depo-subQ at 3-month intervals. We used an elevation of serum progesterone ≥4.7 ng/mL as a surrogate for ovulation and non-parametric statistical methods to assess pharmacokinetic and pharmacodynamic relationships.
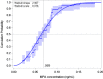
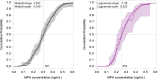
Results: This analysis included 101 women with body mass index (BMI) 18 to 34 kg/m2. Return of ovulation occurred at a median MPA concentration of 0.07 ng/mL (95% CI: 0.06-0.08) and the 90th percentile was 0.10 ng/mL (95% CI: 0.09-0.14). Neither age, race, nor BMI significantly influenced this relationship. The estimated probabilities of ovulation within 4 months of a 104 mg subcutaneous injection and within 7 months of a 150 mg subcutaneous injection (6 plus a 1-month grace) were each below 2.2%.
Conclusions: The typical MPA concentration associated with loss of ovulation suppression is substantially less than the commonly cited threshold of 0.2 ng/mL. Based on our results, MPA levels would rarely be low enough to permit ovulation if the Depo-subQ reinjection interval were extended to four months or if 150 mg Depo-Provera were injected subcutaneously every 6 months.
Implications: Extending the three-month Depo-subQ reinjection interval by one month would result in a 25% reduction in yearly MPA exposure, with little risk of pregnancy. Off-label subcutaneous administration of 150 mg Depo-Provera every 6 months would be a highly effective repurposing of an excellent product, with a similar reduction in cumulative exposure.
Keywords: DMPA; Depo-Provera; Depo-subQ Provera; Pharmacodynamics; Pharmacokinetics.
© 2022 The Author(s). Published by Elsevier Inc.
Figures
Similar articles
-
Suppression of ovulation and pharmacokinetics following subcutaneous administration of various doses of Depo-Provera®: a randomized trial.Contracept X. 2021 Oct 2;3:100070. doi: 10.1016/j.conx.2021.100070. eCollection 2021. Contracept X. 2021. PMID: 34746745 Free PMC article.
-
Clinical trial to evaluate pharmacokinetics and pharmacodynamics of medroxyprogesterone acetate after subcutaneous administration of Depo-Provera.Fertil Steril. 2021 Apr;115(4):1035-1043. doi: 10.1016/j.fertnstert.2020.11.002. Epub 2021 Jan 21. Fertil Steril. 2021. PMID: 33485608 Free PMC article. Clinical Trial.
-
Pharmacokinetics of subcutaneous depot medroxyprogesterone acetate injected in the upper arm.Contraception. 2014 Jan;89(1):31-5. doi: 10.1016/j.contraception.2013.07.002. Epub 2013 Jul 12. Contraception. 2014. PMID: 23993431
-
[Prolonged-action contraceptives].Akush Ginekol (Mosk). 1987 Sep;(9):7-8. Akush Ginekol (Mosk). 1987. PMID: 3322079 Review. Russian.
-
Medroxyprogesterone Acetate.2025 Feb 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Feb 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 30000346 Free Books & Documents. Review.
Cited by
-
Return to ovulation after Sayana Press is injected every 4 months for one year: Empirical and pharmacokinetic/pharmacodynamic modeling results.Contracept X. 2022 Jul 25;4:100080. doi: 10.1016/j.conx.2022.100080. eCollection 2022. Contracept X. 2022. PMID: 35965654 Free PMC article.
-
Development and validation of a multiplexed assay for the measurement of long-acting hormonal contraceptives in plasma via liquid chromatography-tandem mass spectrometry.J Pharm Biomed Anal. 2023 May 10;228:115321. doi: 10.1016/j.jpba.2023.115321. Epub 2023 Mar 5. J Pharm Biomed Anal. 2023. PMID: 36924631 Free PMC article.
-
"I fear those things": non-uptake of contraceptives, and barriers to use among adolescent girls and young women at high risk of HIV infection in Kampala, Uganda.Front Reprod Health. 2023 Aug 15;5:1198672. doi: 10.3389/frph.2023.1198672. eCollection 2023. Front Reprod Health. 2023. PMID: 37649966 Free PMC article.
-
Depot medroxyprogesterone acetate concentrations in patients with and without the use of antiseizure medications.Contraception. 2024 Jun;134:110418. doi: 10.1016/j.contraception.2024.110418. Epub 2024 Mar 6. Contraception. 2024. PMID: 38452921 Free PMC article.
References
-
- Jain J., Jakimiuk A.J., Bode F.R., Ross D., Kaunitz A.M. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269–275. - PubMed
-
- Dragoman M.V., Gaffield M.E. The safety of subcutaneously administered depot medroxyprogesterone acetate (104 mg/0.65 mL): a systematic review. Contraception. 2016;94:202–215. - PubMed
-
- Kaunitz A.M., Darney P.D., Ross D., Wolter K.D., Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80:7–17. - PubMed
-
- DEPO-SUBQ PROVERA 104® [prescribing information] Division of Pfizer Inc.; New York, NY: 2020. Pharmacia and upjohn. Revised December.
-
- U.S. Food and Drug Administration. Depo-subQ Provera 104 (Medroxyprogesterone acetate) injectable suspension new drug application no.: 021583. Center for Drug Evaluation and Research Clinical Pharmacology and Biopharmaceutics Review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021583s000_ClinPh... (accessed April 25, 2021).
LinkOut - more resources
Full Text Sources

