Clinical status, biochemical profile and management of a single cohort of patients with arginase deficiency
- PMID: 35281666
- PMCID: PMC8898719
- DOI: 10.1002/jmd2.12266
Clinical status, biochemical profile and management of a single cohort of patients with arginase deficiency
Abstract
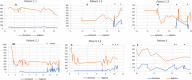
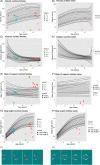
Arginase deficiency is a rare autosomal recessive urea cycle disorder (UCD) caused by mutations in the ARG1 gene encoding arginase that catalyses the hydrolysis of arginine to ornithine and urea. Patients have hyperargininaemia and progressive neurological impairment but generally suffer fewer metabolic decompensations compared to other UCDs. The objective is to describe the clinical features, biochemical profile, neuroradiological findings and experience of managing children with arginase deficiency. Twenty-year retrospective review of patient medical records at a single metabolic centre was performed. Six patients from three unrelated families were identified. Mean age at first symptom was 3.3 (1.5-9.0) years, while mean age at diagnosis was 8.8 (0.16-15.92) years. Four patients developed spastic diplegia and two of six with spastic quadriplegia with classical features including hyperreflexia, clonus and toe walking. This resulted in gait abnormalities that have been monitored using the GAITRite system and required Achilles tendon release in five children. Generalised tonic-clonic seizures and/or absences were present in three of six children and were controlled with anticonvulsants. All patients had moderate learning difficulties. Neuroimaging showed cerebral/cerebellar atrophy in four patients and basal ganglia abnormalities in two. Arginine levels were universally elevated throughout follow-up despite protein restriction, essential amino acid supplementation and ammonia scavengers, and neurological outcome was generally poor. Two patients died following severe metabolic decompensation in adolescence. Children with arginase deficiency continue to present a management challenge of what appears to be an inexorable course of neurocognitive impairment. Further insight into disease mechanisms may provide insight into novel treatment strategies.
Keywords: arginase deficiency; hyperammonaemia; metabolic decompensation; trial end points; urea cycle disorder.
© 2021 The Authors. JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Huemer M, Carvalho DR, Brum JM, et al. Clinical phenotype, biochemical profile, and treatment in 19 patients with arginase 1 deficiency. J Inherit Metab Dis. 2016;39(3):331‐340. - PubMed
-
- Schlune A, Vom Dahl S, Haussinger D, Ensenauer R, Mayatepek E. Hyperargininemia due to arginase I deficiency: the original patients and their natural history, and a review of the literature. Amino Acids. 2015;47(9):1751‐1762. - PubMed
-
- Tsang JP, Poon WL, Luk HM, et al. Arginase deficiency with new phenotype and a novel mutation: contemporary summary. Pediatr Neurol. 2012;47(4):263‐269. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

