Clinical Evidence on the Use of Chinese Herbal Medicine for Acute Infectious Diseases: An Overview of Systematic Reviews
- PMID: 35281902
- PMCID: PMC8914111
- DOI: 10.3389/fphar.2022.752978
Clinical Evidence on the Use of Chinese Herbal Medicine for Acute Infectious Diseases: An Overview of Systematic Reviews
Abstract
Background: Acute infectious diseases constitute the most prevalent public health emergency (PHE) in China. Chinese herbal medicine (CHM) has long been used in the treatment of acute infections, but the overall evidence of its benefit and harm has not been comprehensively and systematically evaluated. Methods: We searched CBM, CNKI, Wanfang, PubMed, Cochrane Library, embase and preprint platforms to retrieve systematic reviews (SRs) on CHM for acute infectious. Participants with COVID-19, SARS, H1N1, tuberculosis, bacillary dysentery, mumps, herpangina, hand-foot-and-mouth disease (HFMD), and other acute infectious diseases were included. Interventional group consisting of patients treated with CHM combined with Western medicine or CHM alone. The AMSTAR 2 tool was used to assess the methodological quality of the retrieved studies. Information on interventions, control measures and outcomes of the included studies was extracted, and meta-analyses were qualitatively synthesized. Results: A total of 51 SRs and meta-analyses were eligible for this overview, including 19 for COVID-19, 11 for hand-foot-and-mouth disease, 8 for severe acute respiratory syndrome (SARS), 4 for tuberculosis, 3 for mumps, 2 for bacillary dysentery, 2 for H1N1 influenza and 2 for herpangina. Six systematic reviews were of high quality, all of which were on the use of CHM for COVID-19; 24 were of moderate quality; 10 were of low quality; and 11 were of very low quality. CHM appeared to have potential benefits in improving clinical symptoms and signs for most infections with an acceptable safety profile, and the clinical evidence of the benefits of CHM for acute respiratory infections such as COVID-19, SARS and H1N1 seems more sufficient than that for other acute infections. Conclusion: Overall, CHM, both decoction and Chinese patent medicine, used alone or in combination with conventional medicine may offer potential benefits to relieving symptoms of people with acute respiratory infections. Full reporting of disease typing, staging, and severity, and intervention details is further required for a better evidence translation to the responses for PHE. Future CHM research should focus mainly on the specific aspects of respiratory infections such as its single use for mild infections, and the adjunct administration for sever infections, and individual CHM prescriptions for well-selected outcomes should be prioritized.
Keywords: COVID-19; Chinese herbal medicine; acute infectious diseases; overview of systematic reviews; public health emergency.
Copyright © 2022 Luo, Zhang, Li, Ren, Liu, Liu, Zhang, Kuang, Cai, Chen and Ni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
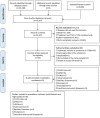
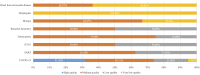
References
-
- Boutron I., Altman D. G., Moher D., Schulz K. F., Ravaud P., Consort Npt Group. (2017). CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann. Intern. Med. 167 (1), 40–47. 10.7326/M17-0046 - DOI - PubMed
-
- Cheng C. W., Wu T. X., Shang H. C., Li Y. P., Altman D. G., Moher D., et al. (2017). CONSORT Extension for Chinese Herbal Medicine Formulas 2017: Recommendations, Explanation, and Elaboration (Simplified Chinese Version). Ann. Intern. Med. 167 (2), W21–W34. 10.7326/IsTranslatedFrom_M17-2977_2 - DOI - PubMed
-
- Ding J., Zhang J., Tian Y., Liu D., Li X. (2013). Meta-analysis of Xiyanping Injection in the Treatment of Hand-Foot-Mouth Disease. Glob. Traditional Chin. Med. 6 (8), 585–588. 10.3969/j.issn.1674-1749.2013.08.007 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

