The Rising Era of "Immunoporosis": Role of Immune System in the Pathophysiology of Osteoporosis
- PMID: 35282271
- PMCID: PMC8906861
- DOI: 10.2147/JIR.S351918
The Rising Era of "Immunoporosis": Role of Immune System in the Pathophysiology of Osteoporosis
Abstract
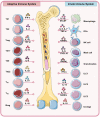
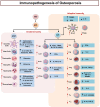
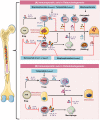
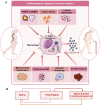
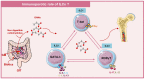
Discoveries in the last few years have emphasized the existence of an enormous breadth of communication between bone and the immune system in maintaining skeletal homeostasis. Originally, the discovery of various factors was assigned to the immune system viz. interleukin (IL)-6, IL-10, IL-17, tumor necrosis factor (TNF)-α, receptor activator of nuclear factor kappa B ligand (RANKL), nuclear factor of activated T cells (NFATc1), etc., but now these factors have also been shown to have a significant impact on osteoblasts (OBs) and osteoclasts (OCs) biology. These discoveries led to an alteration in the approach for the treatment of several bone pathologies including osteoporosis. Osteoporosis is an inflammatory bone anomaly affecting more than 500 million people globally. In 2018, to highlight the importance of the immune system in the pathophysiology of osteoporosis, our group coined the term "immunoporosis". In the present review, we exhaustively revisit the characteristics, mechanism of action, and function of both innate and adaptive immune cells with the goal of understanding the potential of immune cells in osteoporosis. We also highlight the Immunoporotic role of gut microbiota (GM) for the treatment and management of osteoporosis. Importantly, we further discuss whether an immune cell-based strategy to treat and manage osteoporosis is feasible and relevant in clinical settings.
Keywords: adaptive immune cells; bone cells; gut microbiota; immunoporosis; innate immune cells; osteoporosis.
© 2022 Srivastava and Sapra.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures


References
-
- Dobbs MB, Buckwalter J, Saltzman C. Osteoporosis: the increasing role of the orthopaedist. Iowa Orthop J. 1999;19:43–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10847516 - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

