New Insights in Central Venous Disorders. The Role of Transvenous Lead Extractions
- PMID: 35282352
- PMCID: PMC8904723
- DOI: 10.3389/fcvm.2022.783576
New Insights in Central Venous Disorders. The Role of Transvenous Lead Extractions
Abstract
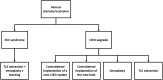
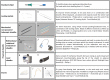
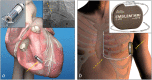
Over the last decades, the implementation of new technology in cardiac pacemakers and defibrillators as well as the increasing life expectancy have been associated with a higher incidence of transvenous lead complications over time. Variable degrees of venous stenosis at the level of the subclavian vein, the innominate trunk and the superior vena cava are reported in up to 50% of implanted patients. Importantly, the number of implanted leads seems to be the main risk factor for such complications. Extraction of abandoned or dysfunctional leads is a potential solution to overcome venous stenosis in case of device upgrades requiring additional leads, but also, in addition to venous angioplasty and stenting, to reduce symptoms related to the venous stenosis itself, i.e., the superior vena cava syndrome. This review explores the role of transvenous lead extraction procedures as therapeutical option in case of central venous disorders related to transvenous cardiac leads. We also describe the different extraction techniques available and other clinical indications for lead extractions such as lead infections. Finally, we discuss the alternative therapeutic options for cardiac stimulation or defibrillation in case of chronic venous occlusions that preclude the implant of conventional transvenous cardiac devices.
Keywords: leadless cardiac pacemaker; subcutaneous cardioverter defibrillator; superior vena cava syndrome; transvenous lead extractions; venous stenosis.
Copyright © 2022 Domenichini, Le Bloa, Carroz, Graf, Herrera-Siklody, Teres, Porretta, Pascale and Pruvot.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bongiorni MG, Burri H, Deharo JC, Starck C, Kennergren C, Saghy L, et al. . 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace. (2018) 20:1217. 10.1093/europace/euy050 - DOI - PubMed
-
- Raatikainen MJ, Arnar DO, Merkely B, Camm AJ, Hindricks G. Access to and clinical use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2016 Report from the European Heart Rhythm Association. Europace. (2016) 18(Suppl. 3):iii1–79. 10.1093/europace/euw244 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

