Comparison of safety and immunogenicity of CoronaVac and ChAdOx1 against the SARS-CoV-2 circulating variants of concern (Alpha, Delta, Beta) in Thai healthcare workers
- PMID: 35282410
- PMCID: PMC8896862
- DOI: 10.1016/j.jvacx.2022.100153
Comparison of safety and immunogenicity of CoronaVac and ChAdOx1 against the SARS-CoV-2 circulating variants of concern (Alpha, Delta, Beta) in Thai healthcare workers
Abstract
Background: Inactivated vaccine (CoronaVac) and chimpanzee adenovirus-vector vaccine (ChAdOx1) have been widely used in resource-limited settings. However, the information on the reactogenicity and immunogenicity of these two vaccines in the same setting are limited.
Methods: Healthy health care workers (HCWs) aged 18 years or older were randomly assigned to receive either two doses of CoronaVac at 4 weeks interval or two doses of ChAdOx1 at 10 weeks interval. Self-reported adverse events (AEs) were collected for 7 days following each vaccination. Immunogenicity was determined by IgG antibodies levels against receptor binding domain (RBD) of the SARS-CoV-2 spike protein (S1 subunit) and the 50% plaque reduction neutralization titers against various strains.
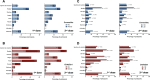
Results: Of the 360 HCWs, 180 in each vaccine group, the median (interquartile range: IQR) age was 35 (29-44) years old and 84.2% were female. Participants who received ChAdOx1 reported higher frequency of AEs than those received CoronaVac after both the first dose (84.4% vs. 66.1%, P < 0.001) and second dose (75.6% vs. 60.6%, P = 0.002), with more AEs in those younger than 30 years of age for both vaccines. The seroconversion rates were 75.6% and 100% following the first dose of CoronaVac and ChAdOx1, respectively. All participants were seropositive at 2 weeks after the second dose. The anti-SARS-CoV-2 RBD IgG levels induced by CoronaVac was lower than ChAdOX1 with geometric means of 164.4 and 278.5 BAU/mL, respectively (P = 0.0066). Both vaccines induced similar levels of neutralizing antibodies against the Wuhan strain, with the titers of 337.4 and 331.2; however, CoronaVac induced significantly lower GMT against Alpha (23.1 vs. 92.5), Delta (21.2 vs. 69.7), and Beta (10.2 vs. 43.6) variants, respectively.
Conclusion: CoronaVac induces lower measurable antibodies against circulating variants but with lower frequency of AEs than ChAdOx1. An earlier boosting to prevent breakthrough infections may be needed.
Keywords: ChAdOx1; CoronVac; Heath care workers; Immunogenicity; Thailand; Variant of concern.
© 2022 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

