Triggering receptor expressed on myeloid cells-2 promotes survival of cardiomyocytes after myocardial ischemic injury through PI3K/AKT pathway
- PMID: 35282669
- PMCID: PMC8898683
- DOI: 10.21037/cdt-21-490
Triggering receptor expressed on myeloid cells-2 promotes survival of cardiomyocytes after myocardial ischemic injury through PI3K/AKT pathway
Abstract
Background: Previous studies have already revealed that triggering receptor expressed on myeloid cells-2 (TREM2) plays a significant protective role during the pathogenesis of ischemia injury in both brain and liver. This study aims to investigate the effect of TREM2 in myocardial ischemic injury.
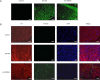
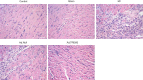
Methods: The mice myocardial infarction (MI) model was established via left anterior descending coronary artery ligation. TREM2 expression was examined with RT-PCR and Western blot. Whereafter, mice were randomly divided into control, sham, MI, Ad.TREM2 transfection group and Ad.Null transfection group. Recombinant adenovirus containing the gene coding full-length mouse TREM2 and EGFP (Ad.TREM2) or control vector containing EGFP gene only (Ad.Null) were immediately intramyocardial injected after left anterior descending ligated. After 7 days of MI, HE, Masson and TUNEL staining were performed to find the myocardial injury, infarcted size and cell apoptosis. Besides, echocardiography was performed to determine cardiac function. In addition, Western blot was performed to check the activity of PI3K/AKT signaling pathway in myocardial tissue. Furthermore, the plasma concentrations of TREM2 in 19 coronary artery disease (CAD) patients and 8 healthy controls were measured.
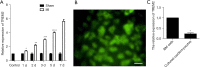
Results: Compared with the sham group, TREM2 expression was significantly up-regulated in cardiac tissue in mice with MI. Cardiac tissue in mice transfected with Ad.TREM2 was demonstrated with alleviated injury, reduced infarct size, and decreased number of apoptotic cells. Echocardiography revealed that heart function was significantly improved in Ad.TREM2 transfection mice. Also, TREM2 transfection significantly activated the phosphorylation of AKT. At last, the plasma concentration of TREM2 was significantly elevated in patients with CAD and correlated with the severity of CAD.
Conclusions: TREM2 may curb myocardial ischemia injury via activating PI3K/AKT signal pathway. Besides, plasma TREM2 may be treated as a potential biomarker in the diagnosis of CAD to reflect the severity of coronary stenosis.
Keywords: Triggering receptor expressed on myeloid cells-2 (TREM2); apoptosis; cardiac function; myocardial ischemic injury.
2022 Cardiovascular Diagnosis and Therapy. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-490/coif). The authors have no conflicts of interest to declare.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous
