Targeting lipophagy in macrophages improves repair in multiple sclerosis
- PMID: 35282773
- PMCID: PMC9629102
- DOI: 10.1080/15548627.2022.2047343
Targeting lipophagy in macrophages improves repair in multiple sclerosis
Abstract
Foamy macrophages containing abundant intracellular myelin remnants are an important pathological hallmark of multiple sclerosis. Reducing the intracellular lipid burden in foamy macrophages is considered a promising therapeutic strategy to induce a phagocyte phenotype that promotes central nervous system repair. Recent research from our group showed that sustained intracellular accumulation of myelin-derived lipids skews these phagocytes toward a disease-promoting and more inflammatory phenotype. Our data now demonstrate that disturbed lipophagy, a selective form of autophagy that helps with the degradation of lipid droplets, contributes to the induction of this phenotype. Stimulating autophagy using the natural disaccharide trehalose reduced the lipid load and inflammatory phenotype of myelin-laden macrophages. Importantly, trehalose was able to boost remyelination in the ex vivo brain slice model and the in vivo cuprizone-induced demyelination model. In summary, our results provide a molecular rationale for impaired metabolism of myelin-derived lipids in macrophages, and identify lipophagy induction as a promising treatment strategy to promote remyelination.Abbreviations: Baf: bafilomycin a1; BMDM: bone marrow-derived macrophage; CD68: CD68 antigen; CNS: central nervous system; LD: lipid droplet; LIPE/HSL: lipase, hormone sensitive; LPS: lipopolysaccharide; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; MBP: myelin basic protein; MGLL: monoglyceride lipase; MS: multiple sclerosis; NO: nitric oxide; NOS2/iNOS: nitric oxide synthase 2, inducible; ORO: oil red o; PNPLA2: patatin-like phospholipase domain containing 2; PLIN2: perilipin 2; TEM: transmission electron microscopy; TFEB: transcription factor EB; TOH: trehalose.
Keywords: Lipid droplets; lipophagy; multiple sclerosis; phagocyte; remyelination.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
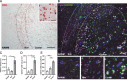
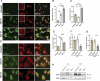




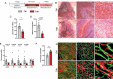

References
-
- Boven LA, Van Meurs M, Van Zwam M, et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129(2):517–526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
