Updates on the Diagnosis and Management of Cold Autoimmune Hemolytic Anemia
- PMID: 35282954
- PMCID: PMC9088174
- DOI: 10.1016/j.hoc.2021.11.001
Updates on the Diagnosis and Management of Cold Autoimmune Hemolytic Anemia
Abstract
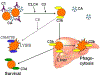
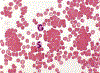
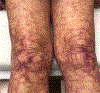
Cold agglutinin disease represents a form of immune-mediated hemolytic anemia whereby an IgM protein either monoclonal or polyclonal deposits complement on the surface of the red blood cell. Once complement is deposited, the 3rd component of complement is recognized by receptors in the mononuclear phagocyte system resulting in spherocytic extravascular hemolysis. This results in a Coombs positive hemolytic anemia with the peripheral blood film showing agglutination. In many instances, the source is a clonal population of lymphoplasmacytic cells in the bone marrow producing a monoclonal IgM protein. Traditional and emerging therapies directed against the production of the IgM may have a positive effect on hemolytic anemia. Success in the management of cold agglutinin disease with rituximab, fludarabine, bortezomib, and bendamustine has all been reported. Recent studies have demonstrated that the blockade of complement with sutimlimab can stop the hemolysis without the use of systemic chemotherapy.
Keywords: Cold agglutinin disease; IgM monoclonal gammopathy; Immune-mediated hemolysis; Waldenström macroglobulinemia.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTEREST
Honorarium from Sanofi.
There are currently no FDA-approved therapies for cold agglutinin hemolytic anemia. All medications mentioned are all off-label.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources