Spatial Coherence in Medical Ultrasound: A Review
- PMID: 35282988
- PMCID: PMC9067166
- DOI: 10.1016/j.ultrasmedbio.2022.01.009
Spatial Coherence in Medical Ultrasound: A Review
Abstract
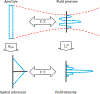
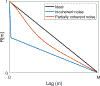
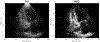
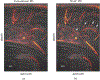
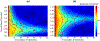
Traditional pulse-echo ultrasound imaging heavily relies on the discernment of signals based on their relative magnitudes but is limited in its ability to mitigate sources of image degradation, the most prevalent of which is acoustic clutter. Advances in computing power and data storage have made it possible for echo data to be alternatively analyzed through the lens of spatial coherence, a measure of the similarity of these signals received across an array. Spatial coherence is not currently explicitly calculated on diagnostic ultrasound scanners but a large number of studies indicate that it can be employed to describe image quality, to adaptively select system parameters and to improve imaging and target detection. With the additional insights provided by spatial coherence, it is poised to play a significant role in the future of medical ultrasound. This review details the theory of spatial coherence in pulse-echo ultrasound and key advances made over the last few decades since its introduction in the 1980s.
Keywords: Beamforming; Clutter reduction; Image quality characterization; Spatial coherence; Tissue characterization.
Copyright © 2022 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures
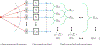








References
-
- Adler L, Hiedemann EA. Determination of the nonlinearity parameter B/A for water and m-xylene. J Acoust Soc Am 1962;34:410–412.
-
- Anderson ME, Soo MSC, Trahey GE. Microcalcifications as elastic scatterers under ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 1998;45:925–934. - PubMed
-
- Asl BM, Mahloojifar A. Minimum variance beamforming combined with adaptive coherence weighting applied to medical ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2009;56:1923–1931. - PubMed
-
- Bamber JC, Hill CR. Acoustic properties of normal and cancerous human liver-I. Dependence on pathological condition. Ultrasound Med Biol 1981;7:121–133. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

