Discovery of farnesoid X receptor and its role in bile acid metabolism
- PMID: 35283218
- PMCID: PMC9038687
- DOI: 10.1016/j.mce.2022.111618
Discovery of farnesoid X receptor and its role in bile acid metabolism
Abstract
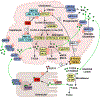
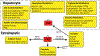
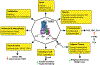
In 1995, the nuclear hormone orphan receptor farnesoid X receptor (FXR, NR1H4) was identified as a farnesol receptor expressed mainly in liver, kidney, and adrenal gland of rats. In 1999, bile acids were identified as endogenous FXR ligands. Subsequently, FXR target genes involved in the regulation of hepatic bile acid synthesis, secretion, and intestinal re-absorption were identified. FXR signaling was proposed as a mechanism of feedback regulation of the rate-limiting enzyme for bile acid synthesis, cholesterol 7⍺-hydroxylase (CYP7A1). The primary bile acids synthesized in the liver are transformed to secondary bile acids by the gut microbiota. The gut-to-liver axis plays a critical role in the regulation of bile acid synthesis, composition and circulating bile acid pool size, which in turn regulates glucose, lipid, and energy metabolism. Dysregulation of bile acid metabolism and FXR signaling in the gut-to-liver axis contributes to metabolic diseases including obesity, diabetes, and non-alcoholic fatty liver disease. This review will cover the discovery of FXR as a bile acid sensor in the regulation of bile acid metabolism and as a metabolic regulator of lipid, glucose, and energy homeostasis. It will also provide an update of FXR functions in the gut-to-liver axis and the drug therapies targeting bile acids and FXR for the treatment of liver metabolic diseases.
Keywords: Bile acid receptors; Cholestasis; FXR; Fatty liver diseases; Metabolic disease.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures

References
-
- Brunt EM, Pathology of nonalcoholic fatty liver disease, Nat Rev Gastroenterol Hepatol 7(4) (2010) 195–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

