Durability of transgene expression after rAAV gene therapy
- PMID: 35283274
- PMCID: PMC9077371
- DOI: 10.1016/j.ymthe.2022.03.004
Durability of transgene expression after rAAV gene therapy
Abstract
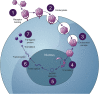
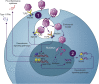
Recombinant adeno-associated virus (rAAV) gene therapy has the potential to transform the lives of patients with certain genetic disorders by increasing or restoring function to affected tissues. Following the initial establishment of transgene expression, it is unknown how long the therapeutic effect will last, although animal and emerging human data show that expression can be maintained for more than 10 years. The durability of therapeutic response is key to long-term treatment success, especially since immune responses to rAAV vectors may prevent re-dosing with the same therapy. This review explores the non-immunological and immunological processes that may limit or improve durability and the strategies that can be used to increase the duration of the therapeutic effect.
Keywords: animals; gene expression; gene expression regulation; gene therapy; gene transfer techniques; genetic therapy/methods; genetic vectors; human genetics; transgenes.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.I.L., M.S., and D.M. are employees of Pfizer Inc. and may own stock/options in the company. M.M. and G.G declare no competing interests.
Figures



References
-
- Sehara Y., Fujimoto K.I., Ikeguchi K., Katakai Y., Ono F., Takino N., Ito M., Ozawa K., Muramatsu S.I. Persistent expression of dopamine-synthesizing enzymes 15 years after gene transfer in a primate model of Parkinson's disease. Hum. Gene Ther. Clin. Dev. 2017;28:74–79. - PubMed
-
- Nathwani A.C., Reiss U., Tuddenham E., Chowdary P., McIntosh J., Riddell A., Pie J., Mahlangu J.N., Recht M., Shen Y.-M., et al. Adeno-associated mediated gene transfer for Hemophilia B: 8 year follow up and impact of removing “empty viral particles” on safety and efficacy of gene transfer [abstract] Blood. 2018;132:491.
-
- Gaudet D., Stroes E.S., Methot J., Brisson D., Tremblay K., Bernelot Moens S.J., Iotti G., Rastelletti I., Ardigo D., Corzo D., et al. Long-term retrospective analysis of gene therapy with alipogene tiparvovec and its effect on lipoprotein lipase deficiency-induced pancreatitis. Hum. Gene Ther. 2016;27:916–925. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

