COVID-19 vaccination among pregnant people in the United States: a systematic review
- PMID: 35283351
- PMCID: PMC8908633
- DOI: 10.1016/j.ajogmf.2022.100616
COVID-19 vaccination among pregnant people in the United States: a systematic review
Abstract
Objective: Pregnant people are at increased risk of COVID-19-related morbidity and mortality, and vaccination presents an important strategy for preventing negative outcomes. However, pregnant people were not included in vaccine trials, and there are limited data on COVID-19 vaccines during pregnancy. The objectives of this systematic review were to identify the safety, immunogenicity, effectiveness, and acceptance of COVID-19 vaccination among pregnant people in the United States.
Data sources: Four databases (PubMed, Web of Science, CINAHL, and Google Scholar) were used to identify eligible studies published from January 1, 2020 through February 6, 2022.
Study eligibility criteria: Inclusion criteria were peer-reviewed empirical research conducted in the United States, publications in English, and research addressing 1 of the following topics: safety, immunogenicity, effectiveness, and acceptance of COVID-19 vaccination among pregnant people.
Methods: A narrative synthesis approach was used to synthesize findings. Critical appraisal was done using the JBI (formerly Joanna Briggs Institute) tool.
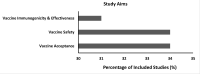
Results: Thirty-two studies were identified. Most studies (n=24) reported the use of Pfizer and Moderna COVID-19 vaccines among pregnant people; only 6 reported the Janssen vaccine. Of the 32 studies, 11 examined COVID-19 vaccine safety, 10 investigated immunogenicity and effectiveness, and 11 assessed vaccine acceptance among pregnant people. Injection-site pain and fatigue were the most common adverse events. One case study reported immune thrombocytopenia. COVID-19 vaccination did not increase the risk of adverse pregnancy or neonatal outcomes compared with unvaccinated pregnant people. After COVID-19 vaccination, pregnant people had a robust immune response, and vaccinations conferred protective immunity to newborns through breast milk and placental transfer. COVID-19 vaccine acceptance was low among pregnant people in the United States. African American race, Hispanic ethnicity, younger age, low education, previous refusal of the influenza vaccine, and lack of provider counseling were associated with low vaccine acceptance.
Conclusion: Peer-reviewed studies support COVID-19 vaccine safety and protective effects on pregnant people and their newborns. Future studies that use rigorous methodologies and include diverse populations are needed to confirm current findings. In addition, targeted and tailored strategies are needed to improve vaccine acceptance, especially among minorities.
Keywords: COVID-19 vaccine; immunogenicity; messenger RNA vaccine; neonatal outcomes; pregnancy; pregnancy outcomes; vaccine acceptance; vaccine effectiveness; vaccine hesitancy; vaccine safety.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

