Development and Multicentre Validation of the Modena Score to Predict Survival in Advanced Biliary Cancers Undergoing Second-Line Chemotherapy
- PMID: 35283642
- PMCID: PMC8906899
- DOI: 10.2147/CMAR.S346235
Development and Multicentre Validation of the Modena Score to Predict Survival in Advanced Biliary Cancers Undergoing Second-Line Chemotherapy
Abstract
Background: The role of second-line chemotherapy in advanced biliary cancers (ABCs) has only recently been established in phase III randomized trial and the optimal selection of patients most likely to benefit from it remains challenging.
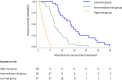
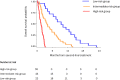
Methods: A cohort of 98 ABC treated second-line chemotherapy was used as a developmental dataset to identify covariates independently associated with overall survival (OS). Kaplan-Meier analysis was used to investigate the association between variables and OS and those retaining statistically significance were combined in a multiplexed score.
Results: The following pretreatment variables were independently associated with OS: ECOG PS > 0, peritoneal disease, LDH > 430 UI/L, albumin <3.5 gr/dL, gamma-GT >100 UI/L, sodium <140 mEq/L, absolute lymphocyte count <1000/mmc, and PFS to first-line <6 months. Based on these results, a scoring system was developed that identified three subgroups with statistically different OS: low-risk (mOS 18 months), intermediate-risk (mOS 9.4 months) and high-risk (mOS 2.9 months) (p < 0.001). The prognostic model was both internally and externally validated in a multicentre cohort of 120 ABCs.
Conclusion: The Modena score is a multiplexed scoring system capable of accurately risk-stratified ABCs treated with second-line chemotherapy. Based on its reproducibility, usability and generalizability, it has the potential for assisting therapeutic decision-making in the clinic and risk-stratification in future trials.
Keywords: biliary tract cancer; chemotherapy; prognostic score; second-line; survival.
© 2022 Salati et al.
Conflict of interest statement
Dr Luigi Marcheselli reports scientific consultant for Sandoz Spa during Jan 2021–Sep 2022, free of fee. Dr Fabio Gelsomino reports personal fees from Servier, Iqvia, Eli Lilly, Merck Serono, and Amgen, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
The A.L.A.N. score identifies prognostic classes in advanced biliary cancer patients receiving first-line chemotherapy.Eur J Cancer. 2019 Aug;117:84-90. doi: 10.1016/j.ejca.2019.05.030. Epub 2019 Jul 2. Eur J Cancer. 2019. PMID: 31276980
-
The prognostic nutritional index predicts survival and response to first-line chemotherapy in advanced biliary cancer.Liver Int. 2020 Mar;40(3):704-711. doi: 10.1111/liv.14314. Epub 2019 Dec 11. Liver Int. 2020. PMID: 31773848
-
Prognostic score for second-line chemotherapy of advanced non-small-cell lung cancer: external validation in a phase III trial comparing vinflunine with docetaxel.Lung Cancer. 2012 Jul;77(1):116-20. doi: 10.1016/j.lungcan.2012.01.013. Epub 2012 Feb 21. Lung Cancer. 2012. PMID: 22361218
-
Combination versus mono-therapy as salvage treatment for advanced biliary tract cancer: A comprehensive meta-analysis of published data.Crit Rev Oncol Hematol. 2019 Jul;139:134-142. doi: 10.1016/j.critrevonc.2019.01.001. Epub 2019 Jan 4. Crit Rev Oncol Hematol. 2019. PMID: 30979533 Review.
-
Systemic treatment in advanced biliary cancers: A multicenter Australian analysis and review.Asia Pac J Clin Oncol. 2017 Oct;13(5):e291-e297. doi: 10.1111/ajco.12638. Epub 2016 Nov 10. Asia Pac J Clin Oncol. 2017. PMID: 27860270 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

