Bevacizumab is an Efficient Therapeutic Approach with Low Side Effects in Patient-Derived Xenografts of Adenoid Cystic Carcinoma of the Lacrimal Gland
- PMID: 35283648
- PMCID: PMC8912937
- DOI: 10.2147/CMAR.S352623
Bevacizumab is an Efficient Therapeutic Approach with Low Side Effects in Patient-Derived Xenografts of Adenoid Cystic Carcinoma of the Lacrimal Gland
Abstract
Purpose: Adenoid cystic carcinoma (ACC) of the lacrimal gland (LGACC) is an aggressive malignant lacrimal gland tumor with a generally poor prognosis. Survival rates for LGACC are 56% at 5 years and 49% at 10 years. Recent studies have indicated that anti-vascular endothelial growth factor (VEGF) therapy can inhibit angiogenesis in ACC cells. This study was designed to explore the efficacy of the antiangiogenic drug bevacizumab in a LGACC patient-derived xenograft (PDX) animal model.
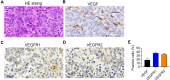
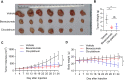
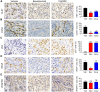
Methods: The histological structure of PDX was determined by hematoxylin-eosin staining to confirm successful xenografting. Immunohistochemistry (IHC) was used to detect the expression of neovascularization-related genes in LGACC patients and in the PDX model, including VEGF, VEGFR1, and FGFR. In order to compare the efficacy of antiangiogenic drug and traditional chemotherapy drug, PDX models were treated with bevacizumab and cisplatin respectively, and body weight was evaluated. Subsequently, the neovascularization-related proteins VEGF, VEGFR2, and CD34, tumor suppressor P53 and proliferation-related protein Ki67 were analyzed by IHC. Quantitative real-time PCR was employed to examine the mRNA expression of apoptosis-related genes BAD and Caspase 9, and of HIF1α.
Results: VEGF, VEGFR1, and FGFR were highly expressed in patients with LGACC and PDX models. Both bevacizumab and cisplatin treatment inhibited PDX tumor growth. The body weight of PDX models treated with cisplatin significantly decreased from day 15, while those treated with bevacizumab did not markedly change. Bevacizumab reduced the expression of VEGF, CD34, and Ki67 in PDX tumors; whereas, bevacizumab upregulated P53 and downregulated HIF1α levels.
Conclusion: The present study indicates that antiangiogenic drugs may be a promising treatment strategy for LGACC.
Keywords: LGACC; PDX; VEGF; bevacizumab; cisplatin.
© 2022 Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures


Similar articles
-
Spatial Transcriptomics Identifies Expression Signatures Specific to Lacrimal Gland Adenoid Cystic Carcinoma Cells.Cancers (Basel). 2023 Jun 16;15(12):3211. doi: 10.3390/cancers15123211. Cancers (Basel). 2023. PMID: 37370820 Free PMC article.
-
Neoadjuvant Intra-arterial Cytoreductive Chemotherapy Improves Outcomes in Lacrimal Gland Adenoid Cystic Carcinoma.Oncologist. 2024 Mar 4;29(3):263-269. doi: 10.1093/oncolo/oyad346. Oncologist. 2024. PMID: 38227581 Free PMC article.
-
Expression of survivin in adenoid cystic carcinoma of the lacrimal gland and the effect of intervention with arsenic trioxide in vitro.Exp Ther Med. 2015 Jul;10(1):330-334. doi: 10.3892/etm.2015.2466. Epub 2015 Apr 30. Exp Ther Med. 2015. PMID: 26170957 Free PMC article.
-
Treatment strategies and prognostic insights for lacrimal gland adenoid cystic carcinoma: a review.Discov Oncol. 2025 May 22;16(1):858. doi: 10.1007/s12672-025-02468-5. Discov Oncol. 2025. PMID: 40402353 Free PMC article. Review.
-
Integration of novel agents in the treatment of colorectal cancer.Cancer Chemother Pharmacol. 2004 Sep;54 Suppl 1:S32-9. doi: 10.1007/s00280-004-0884-0. Cancer Chemother Pharmacol. 2004. PMID: 15309512 Review.
Cited by
-
Vascular Growth Factor Inhibition with Bevacizumab Improves Cardiac Electrical Alterations and Fibrosis in Experimental Acute Chagas Disease.Biology (Basel). 2023 Nov 10;12(11):1414. doi: 10.3390/biology12111414. Biology (Basel). 2023. PMID: 37998013 Free PMC article.
-
Prognostic factors for lacrimal gland adenoid cystic carcinoma: a retrospective study in Chinese patients.Int J Ophthalmol. 2024 Aug 18;17(8):1423-1430. doi: 10.18240/ijo.2024.08.06. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39156780 Free PMC article.
-
Adenoid Cystic Carcinoma of the Lacrimal Gland.J Curr Ophthalmol. 2024 Oct 16;36(1):1-8. doi: 10.4103/joco.joco_231_23. eCollection 2024 Jan-Mar. J Curr Ophthalmol. 2024. PMID: 39553321 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

