Performance of Molecular Mechanics Force Fields for RNA Simulations: Stability of UUCG and GNRA Hairpins
- PMID: 35283696
- PMCID: PMC8916691
- DOI: 10.1021/ct100481h
Performance of Molecular Mechanics Force Fields for RNA Simulations: Stability of UUCG and GNRA Hairpins
Abstract
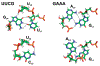
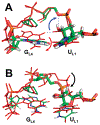
The RNA hairpin loops represent important RNA topologies with indispensable biological functions in RNA folding and tertiary interactions. 5'-UNCG-3' and 5'-GNRA-3' RNA tetraloops are the most important classes of RNA hairpin loops. Both tetraloops are highly structured with characteristic signature three-dimensional features and are recurrently seen in functional RNAs and ribonucleoprotein particles. Explicit solvent molecular dynamics (MD) simulation is a computational technique which can efficiently complement the experimental data and provide unique structural dynamics information on the atomic scale. Nevertheless, the outcome of simulations is often compromised by imperfections in the parametrization of simplified pairwise additive empirical potentials referred to also as force fields. We have pointed out in several recent studies that a force field description of single-stranded hairpin segments of nucleic acids may be particularly challenging for the force fields. In this paper, we report a critical assessment of a broad set of MD simulations of UUCG, GAGA, and GAAA tetraloops using various force fields. First, we utilized the three widely used variants of Cornell et al. (AMBER) force fields known as ff94, ff99, and ff99bsc0. Some simulations were also carried out with CHARMM27. The simulations reveal several problems which show that these force fields are not able to retain all characteristic structural features (structural signature) of the studied tetraloops. Then we tested four recent reparameterizations of glycosidic torsion of the Cornell et al. force field (two of them being currently parametrized in our laboratories). We show that at least some of the new versions show an improved description of the tetraloops, mainly in the syn glycosidic torsion region of the UNCG tetraloop. The best performance is achieved in combination with the bsc0 parametrization of the α/γ angles. Another critically important region to properly describe RNA molecules is the anti/high-anti region of the glycosidic torsion, where there are significant differences among the tested force fields. The tetraloop simulations are complemented by simulations of short A-RNA stems, which are especially sensitive to an appropriate description of the anti/high-anti region. While excessive accessibility of the high-anti region converts the A-RNA into a senseless "ladder-like" geometry, excessive penalization of the high-anti region shifts the simulated structures away from typical A-RNA geometry to structures with a visibly underestimated inclination of base pairs with respect to the helical axis.
Figures
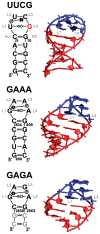


References
-
- Leontis NB; Westhof E Analysis of RNA motifs. Cur. Opin. Struct. Biol 2003, 13, 300–308. - PubMed
-
- Mathews DH; Turner DH Prediction of RNA secondary structure by free energy minimization. Cur. Opin. Struct. Biol 2006, 16, 270–278. - PubMed
-
- Uhlenbeck OC Nucleic-Acid Structure - Tetraloops and RNA Folding. Nature 1990, 346, 613–614. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
