Hyperacute Excitotoxic Mechanisms and Synaptic Dysfunction Involved in Traumatic Brain Injury
- PMID: 35283730
- PMCID: PMC8907921
- DOI: 10.3389/fnmol.2022.831825
Hyperacute Excitotoxic Mechanisms and Synaptic Dysfunction Involved in Traumatic Brain Injury
Abstract
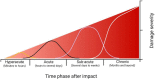
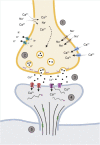
The biological response of brain tissue to biomechanical strain are of fundamental importance in understanding sequela of a brain injury. The time after impact can be broken into four main phases: hyperacute, acute, subacute and chronic. It is crucial to understand the hyperacute neural outcomes from the biomechanical responses that produce traumatic brain injury (TBI) as these often result in the brain becoming sensitized and vulnerable to subsequent TBIs. While the precise physical mechanisms responsible for TBI are still a matter of debate, strain-induced shearing and stretching of neural elements are considered a primary factor in pathology; however, the injury-strain thresholds as well as the earliest onset of identifiable pathologies remain unclear. Dendritic spines are sites along the dendrite where the communication between neurons occurs. These spines are dynamic in their morphology, constantly changing between stubby, thin, filopodia and mushroom depending on the environment and signaling that takes place. Dendritic spines have been shown to react to the excitotoxic conditions that take place after an impact has occurred, with a shift to the excitatory, mushroom phenotype. Glutamate released into the synaptic cleft binds to NMDA and AMPA receptors leading to increased Ca2+ entry resulting in an excitotoxic cascade. If not properly cleared, elevated levels of glutamate within the synaptic cleft will have detrimental consequences on cellular signaling and survival of the pre- and post-synaptic elements. This review will focus on the synaptic changes during the hyperacute phase that occur after a TBI. With repetitive head trauma being linked to devastating medium - and long-term maladaptive neurobehavioral outcomes, including chronic traumatic encephalopathy (CTE), understanding the hyperacute cellular mechanisms can help understand the course of the pathology and the development of effective therapeutics.
Keywords: dendritic spine; excitotoxicity; neurodegeneration; synaptic dysfunction; traumatic brain injury.
Copyright © 2022 Hoffe and Holahan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

