Real-Time Detection and Feedback of Canonical Electroencephalogram Microstates: Validating a Neurofeedback System as a Function of Delay
- PMID: 35283737
- PMCID: PMC8913511
- DOI: 10.3389/fnsys.2022.786200
Real-Time Detection and Feedback of Canonical Electroencephalogram Microstates: Validating a Neurofeedback System as a Function of Delay
Abstract
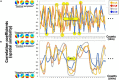
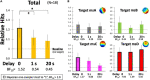
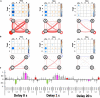
Recent neurotechnology has developed various methods for neurofeedback (NF), in which participants observe their own neural activity to be regulated in an ideal direction. EEG-microstates (EEGms) are spatially featured states that can be regulated through NF training, given that they have recently been indicated as biomarkers for some disorders. The current study was conducted to develop an EEG-NF system for detecting "canonical 4 EEGms" in real time. There are four representative EEG states, regardless of the number of channels, preprocessing procedures, or participants. Accordingly, our 10 Hz NF system was implemented to detect them (msA, B, C, and D) and audio-visually inform participants of its detection. To validate the real-time effect of this system on participants' performance, the NF was intentionally delayed for participants to prevent their cognitive control in learning. Our results suggest that the feedback effect was observed only under the no-delay condition. The number of Hits increased significantly from the baseline period and increased from the 1- or 20-s delay conditions. In addition, when the Hits were compared among the msABCD, each cognitive or perceptual function could be characterized, though the correspondence between each microstate and psychological ability might not be that simple. For example, msD should be generally task-positive and less affected by the inserted delay, whereas msC is more delay-sensitive. In this study, we developed and validated a new EEGms-NF system as a function of delay. Although the participants were naive to the inserted delay, the real-time NF successfully increased their Hit performance, even within a single-day experiment, although target specificity remains unclear. Future research should examine long-term training effects using this NF system.
Keywords: EEG microstates; control; delay; neurofeedback; sense of agency.
Copyright © 2022 Asai, Hamamoto, Kashihara and Imamizu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

