Long-Term Cultivation of Human Atrial Myocardium
- PMID: 35283779
- PMCID: PMC8905341
- DOI: 10.3389/fphys.2022.839139
Long-Term Cultivation of Human Atrial Myocardium
Erratum in
-
Corrigendum: long-term cultivation of human atrial myocardium.Front Physiol. 2023 May 3;14:1206654. doi: 10.3389/fphys.2023.1206654. eCollection 2023. Front Physiol. 2023. PMID: 37206365 Free PMC article.
Abstract
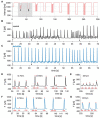
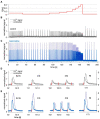
Organotypic culture of human ventricular myocardium is emerging in basic and translational cardiac research. However, few institutions have access to human ventricular tissue, whereas atrial tissue is more commonly available and important for studying atrial physiology. This study presents a method for long-term cultivation of beating human atrial myocardium. After written informed consent, tissues from the right-atrial appendage were obtained from patients with sinus rhythm undergoing open heart surgery with cardiopulmonary bypass. Trabeculae (pectinate muscles) prepared from the samples were installed into cultivation chambers at 37°C with a diastolic preload of 500 μN. After 2 days with 0.5 Hz pacing, stimulation frequency was set to 1 Hz. Contractile force was monitored continuously. Beta-adrenergic response, refractory period (RP) and maximum captured frequency (fmax) were assessed periodically. After cultivation, viability and electromechanical function were investigated, as well as the expression of several genes important for intracellular Ca2+ cycling and electrophysiology. Tissue microstructure was analyzed by confocal microscopy. We cultivated 19 constantly beating trabeculae from 8 patient samples for 12 days and 4 trabeculae from 3 specimen for 21 days. Functional parameters were compared directly after installation (0 d) with those after 12 d in culture. Contraction force was 384 ± 69 μN at 0 d and 255 ± 90 μN at 12 d (p = 0.8, n = 22), RP 480 ± 97 ms and 408 ± 78 ms (p = 0.3, n = 9), fmax 3.0 ± 0.5 Hz and 3.8 ± 0.5 Hz (p = 0.18, n = 9), respectively. Application of 100 nM isoprenaline to 11 trabeculae at 7 d increased contraction force from 168 ± 35 μN to 361 ± 60 μN (p < 0.01), fmax from 6.4 ± 0.6 Hz to 8.5 ± 0.4 Hz (p < 0.01) and lowered RP from 319 ± 22 ms to 223 ± 15 ms. CACNA1c (L-type Ca2+ channel subunit) and GJA1 (connexin-43) mRNA expressions were not significantly altered at 12 d vs 0 d, while ATP2A (SERCA) and KCNJ4 (Kir2.3) were downregulated, and KCNJ2 (Kir2.1) was upregulated. Simultaneous Ca2+ imaging and force recording showed preserved excitation-contraction coupling in cultivated trabeculae. Confocal microscopy indicated preserved cardiomyocyte structure, unaltered amounts of extracellular matrix and gap junctions. MTT assays confirmed viability at 12 d. We established a workflow that allows for stable cultivation and functional analysis of beating human atrial myocardium for up to 3 weeks. This method may lead to novel insights into the physiology and pathophysiology of human atrial myocardium.
Keywords: calcium imaging; confocal microscopy; gene expression; human atrium; in vitro; refractory period; tissue culture.
Copyright © 2022 Klumm, Heim, Fiegle, Weyand, Volk and Seidel.
Conflict of interest statement
TS is shareholder of InVitroSys GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Abu-Khousa M., Fiegle D. J., Sommer S. T., Minabari G., Milting H., Heim C., et al. (2020). The degree of t-system remodeling predicts negative force-frequency relationship and prolonged relaxation time in failing human myocardium. Front. Physiol. 11:182. 10.3389/fphys.2020.00182 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

