Parathyroid Imaging: Past, Present, and Future
- PMID: 35283807
- PMCID: PMC8914059
- DOI: 10.3389/fendo.2021.760419
Parathyroid Imaging: Past, Present, and Future
Abstract
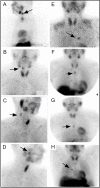
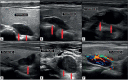
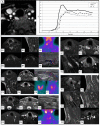
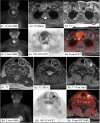
The goal of parathyroid imaging is to identify all sources of excess parathyroid hormone secretion pre-operatively. A variety of imaging approaches have been evaluated and utilized over the years for this purpose. Ultrasound relies solely on structural features and is without radiation, however is limited to superficial evaluation. 4DCT and 4DMRI provide enhancement characteristics in addition to structural features and dynamic enhancement has been investigated as a way to better distinguish parathyroid from adjacent structures. It is important to recognize that 4DCT provides valuable information however results in much higher radiation dose to the thyroid gland than the other available examinations, and therefore the optimal number of phases is an area of controversy. Single-photon scintigraphy with 99mTc-Sestamibi, or dual tracer 99mTc-pertechnetate and 99mTc-sestamibi with or without SPECT or SPECT/CT is part of the standard of care in many centers with availability and expertise in nuclear medicine. This molecular imaging approach detects cellular physiology such as mitochondria content found in parathyroid adenomas. Combining structural imaging such as CT or MRI with molecular imaging in a hybrid approach allows the ability to obtain robust structural and functional information in one examination. Hybrid PET/CT is widely available and provides improved imaging and quantification over SPECT or SPECT/CT. Emerging PET imaging techniques, such as 18F-Fluorocholine, have the exciting potential to reinvent parathyroid imaging. PET/MRI may be particularly well suited to parathyroid imaging, where available, because of the ability to perform dynamic contrast-enhanced imaging and co-registered 18F-Fluorocholine PET imaging simultaneously with low radiation dose to the thyroid. A targeted agent specific for a parathyroid tissue biomarker remains to be identified.
Keywords: 4D CT MR; PET; hyperparathyroidism; oncoradiology; parathyroid adenoma; parathyroid imaging; parathyroidectomy; scintigraphy.
Copyright © 2022 Morris, Saboury, Ahlman, Malayeri, Jones, Chen and Millo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Askanazy M. Ueber Ostitis Deformans Ohne Osteoides Gewebe. Arch Pathanat Inst Tübingen (1904) 4:398–422.
-
- Schmorl G. Ueber Ostitis Deformans Ohne Osteoides Gewebe. Munch Med Wschr (1907) 54:494. doi: 10.25291/VR/1907-VLR-54 - DOI
-
- Molineus L. Über Die Multiplen Braunen Tumoren Bei Osteomalacie. Langenbecks Arch Klin Chir Ver Dtsch Z Chir (1913) 101:333.
-
- Poltauf. Zbl allg path Anat (1913) 24:959.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

