Bovine Animal Model for Studying the Maternal Microbiome, in utero Microbial Colonization and Their Role in Offspring Development and Fetal Programming
- PMID: 35283808
- PMCID: PMC8916045
- DOI: 10.3389/fmicb.2022.854453
Bovine Animal Model for Studying the Maternal Microbiome, in utero Microbial Colonization and Their Role in Offspring Development and Fetal Programming
Abstract
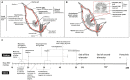
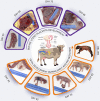
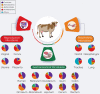
Recent developments call for further research on the timing and mechanisms involved in the initial colonization of the fetal/infant gut by the maternal microbiome and its role in Developmental Origins of Health and Disease (DOHaD). Although progress has been made using primarily preterm infants, ethical and legal constraints hinder research progress in embryo/fetal-related research and understanding the developmental and mechanistic roles of the maternal microbiome in fetal microbial imprinting and its long-term role in early-life microbiome development. Rodent models have proven very good for studying the role of the maternal microbiome in fetal programming. However, some inherent limitations in these animal models make it challenging to study perinatal microbial colonization from a biomedical standpoint. In this review, we discuss the potential use of bovine animals as a biomedical model to study the maternal microbiome, in utero microbial colonization of the fetal gut, and their impact on offspring development and DOHaD.
Keywords: biomedical research; bovine model; developmental origins of health and disease; fetal programming; in utero microbial colonization; maternal microbiome.
Copyright © 2022 Amat, Dahlen, Swanson, Ward, Reynolds and Caton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abecia L., Waddams K. E., Martínez-Fernandez G., Martín-García A. I., Ramos-Morales E., Newbold C. J., et al. (2014). An antimethanogenic nutritional intervention in early life of ruminants modifies ruminal colonization by archaea. Archaea 2014:841463. doi: 10.1155/2014/841463, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

