Transcriptomic and Metabolomic Analyses Reveal the Differential Regulatory Mechanisms of Compound Material on the Responses of Brassica campestris to Saline and Alkaline Stresses
- PMID: 35283897
- PMCID: PMC8905141
- DOI: 10.3389/fpls.2022.820540
Transcriptomic and Metabolomic Analyses Reveal the Differential Regulatory Mechanisms of Compound Material on the Responses of Brassica campestris to Saline and Alkaline Stresses
Abstract
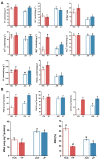
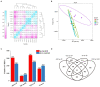
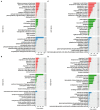
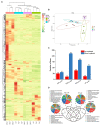
Oilseed rape not only has the function of improve saline and alkaline soils, but also alleviate the local feed shortage. However, medium- and high-degree soil salinization and alkalinization always inhibit the growth of oilseed rape. Studies have shown that compound material can improve the tolerance to saline and alkaline stress of crops, but the difference in the regulation mechanism of compound material on oilseed rape in saline and alkaline soils is not clear. This study explored the difference through determining the leaf ion contents, physiological indexes, transcriptomics, and metabolomics of oilseed rape in salinized soil (NaCl 8 g kg-1) and alkalinized soil (Na2CO3 8 g kg-1) at full flowering stage, respectively after the application of compound material. The results showed that in salinized and alkalinized soil, the compound material upregulated the genes related to the regulation of potassium ion transport, and changed the amino acid metabolic pathway, which reduced the contents of Na+, malondialdehyde (MDA), and relative conductivity (REC) in leaves, and increased the contents of K+ and Mg2+ and the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT). However, there were differences in the regulation mechanism of compound material in salinized and alkalinized soil. In salinized soil, the compound material improved the tolerance of oilseed rape to saline stress by upregulating transcription factors mannose-1-phosphate guanylyltransferase (GPMM) and Glutamine--fructose-6-phosphate transaminase (GFPT) and downregulating phosphomannomutase (PMM) to change nucleotide metabolism pathway and lipid metabolism pathway. In alkalized soil, the compound material improved the tolerance of oilseed rape to alkaline stress by upregulating transcription factors Phenylalanine ammonia lyase (PAL) to change the biosynthesis pathway of other secondary metabolites. Therefore, the compound material can improve the tolerance of oilseed rape to saline and alkaline stress by regulating the genetic adaptability and apparent plasticity, but the mechanisms were different. This study provides a practical method for the ecological environment restoration and the development of animal husbandry.
Keywords: animal husbandry; biosynthesis of other secondary metabolites; forage crop; genetic adaptability; saline–alkali land development.
Copyright © 2022 Li, An, Hong, Chang, Wang and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

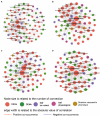
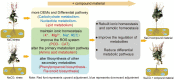
References
-
- Ahmad P. (2012). Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 11, 2694–2703. doi: 10.5897/ABJ11.3203 - DOI
-
- Alvarez-Acosta C., Marrero-Dominguez A., Gallo-Llobet L., Gonzalez-Rodriguez A. M. (2019). Effects of NaCl and NaHCO3 stress on morphological growth and nutrient metabolism on selected avocados. J. Plant Nutr. 42, 164–177. doi: 10.1080/01904167.2018.1551490 - DOI
-
- Ashraf M., Ali Q. (2008). Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (brassica napus L.). Environ. Exp. Bot. 63, 266–273. doi: 10.1016/j.envexpbot.2007.11.008 - DOI
-
- Bao S. D. (2000). Soil Agrochemical Analysis. Beijing, China: China Agriculture Press.
LinkOut - more resources
Full Text Sources
Miscellaneous

