Rehydration Compensation of Winter Wheat Is Mediated by Hormone Metabolism and De-Peroxidative Activities Under Field Conditions
- PMID: 35283926
- PMCID: PMC8908233
- DOI: 10.3389/fpls.2022.823846
Rehydration Compensation of Winter Wheat Is Mediated by Hormone Metabolism and De-Peroxidative Activities Under Field Conditions
Abstract
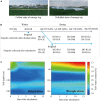
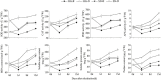
Water deficit and rehydration frequently occur during wheat cultivation. Previous investigations focused on the water deficit and many drought-responsive genes have been identified in winter wheat. However, the hormone-related metabolic responses and de-peroxidative activities associated with rehydration are largely unknown. In this study, leaves of two winter wheat cultivars, "Hengguan35" (HG, drought-tolerant cultivar) and "Shinong086" (SN, drought-sensitive cultivar), were used to investigate water deficit and the post-rehydration process. Rehydration significantly promoted wheat growth and postponed spike development. Quantifications of antioxidant enzymes, osmotic stress-related substances, and phytohormones revealed that rehydration alleviated the peroxidation and osmotic stress caused by water deficit in both cultivars. The wheat cultivar HG showed a better rehydration-compensation phenotype than SN. Phytohormones, including abscisic acid, gibberellin (GA), jasmonic acid (JA), and salicylic acid (SA), were detected using high-performance liquid chromatography and shown to be responsible for the rehydration process. A transcriptome analysis showed that differentially expressed genes related to rehydration were enriched in hormone metabolism- and de-peroxidative stress-related pathways. Suppression of genes associated with abscisic acid signaling transduction were much stronger in HG than in SN upon rehydration treatment. HG also kept a more balanced expression of genes involved in reactive oxygen species pathway than SN. In conclusion, we clarified the hormonal changes and transcriptional profiles of drought-resistant and -sensitive winter wheat cultivars in response to drought and rehydration, and we provided insights into the molecular processes involved in rehydration compensation.
Keywords: de-peroxidative stress; drought stress; hormone metabolism; rehydration compensation; transcriptomic; wheat.
Copyright © 2022 Liu, Wang, Liu, Bao, Hou, Yang and Zhen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Ahmad I., Khaliq I., Mahmood N., Khan N. (2015). Morphological and physiological criteria for drought tolerance at seedling stage in wheat. J. Anim. Plant Sci. 25 1041–1048.
-
- Araya A., Prasad P. V. V., Gowda P. H., Kisekka I., Foster A. J. (2019). Yield and water productivity of winter wheat under various irrigation capacities. J. Am. Water Res. Assoc. 55 24–37. 10.1111/1752-1688.12721 - DOI
-
- Asada Y. N. K. (1980). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22 867–880.
LinkOut - more resources
Full Text Sources

