Metastable dynamics of neural circuits and networks
- PMID: 35284030
- PMCID: PMC8900181
- DOI: 10.1063/5.0062603
Metastable dynamics of neural circuits and networks
Abstract
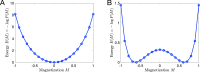
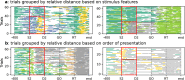
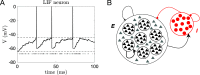
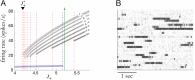
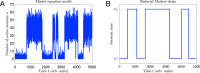
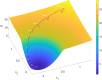
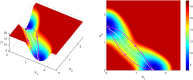
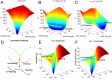
Cortical neurons emit seemingly erratic trains of action potentials or "spikes," and neural network dynamics emerge from the coordinated spiking activity within neural circuits. These rich dynamics manifest themselves in a variety of patterns, which emerge spontaneously or in response to incoming activity produced by sensory inputs. In this Review, we focus on neural dynamics that is best understood as a sequence of repeated activations of a number of discrete hidden states. These transiently occupied states are termed "metastable" and have been linked to important sensory and cognitive functions. In the rodent gustatory cortex, for instance, metastable dynamics have been associated with stimulus coding, with states of expectation, and with decision making. In frontal, parietal, and motor areas of macaques, metastable activity has been related to behavioral performance, choice behavior, task difficulty, and attention. In this article, we review the experimental evidence for neural metastable dynamics together with theoretical approaches to the study of metastable activity in neural circuits. These approaches include (i) a theoretical framework based on non-equilibrium statistical physics for network dynamics; (ii) statistical approaches to extract information about metastable states from a variety of neural signals; and (iii) recent neural network approaches, informed by experimental results, to model the emergence of metastable dynamics. By discussing these topics, we aim to provide a cohesive view of how transitions between different states of activity may provide the neural underpinnings for essential functions such as perception, memory, expectation, or decision making, and more generally, how the study of metastable neural activity may advance our understanding of neural circuit function in health and disease.
© 2022 Author(s).
Figures






References
-
- Cao R., Pastukhov A., Aleshin S., Mattia M., and Braun J., “ Instability with a purpose: How the visual brain makes decisions in a volatile world,” bioRxiv:2020.06.09.142497 (2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
