Versatile, do-it-yourself, low-cost spinning disk confocal microscope
- PMID: 35284165
- PMCID: PMC8884209
- DOI: 10.1364/BOE.442087
Versatile, do-it-yourself, low-cost spinning disk confocal microscope
Abstract
Confocal microscopy is an invaluable tool for 3D imaging of biological specimens, however, accessibility is often limited to core facilities due to the high cost of the hardware. We describe an inexpensive do-it-yourself (DIY) spinning disk confocal microscope (SDCM) module based on a commercially fabricated chromium photomask that can be added on to a laser-illuminated epifluorescence microscope. The SDCM achieves strong performance across a wide wavelength range (∼400-800 nm) as demonstrated through a series of biological imaging applications that include conventional microscopy (immunofluorescence, small-molecule stains, and fluorescence in situ hybridization) and super-resolution microscopy (single-molecule localization microscopy and expansion microscopy). This low-cost and simple DIY SDCM is well-documented and should help increase accessibility to confocal microscopy for researchers.
© 2022 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
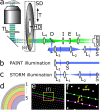
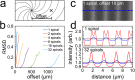



References
-
- Pawley J. B., ed., Handbook of Biological Confocal Microscopy , 3rd ed (Springer, 2006).
-
- Oreopoulos J., Berman R., Browne M., “Spinning-disk confocal microscopy,” in Methods Cell Biol. , 123 (Elsevier, 2014), pp. 153–175. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
