Three-dimensional light-field microendoscopy with a GRIN lens array
- PMID: 35284166
- PMCID: PMC8884202
- DOI: 10.1364/BOE.447578
Three-dimensional light-field microendoscopy with a GRIN lens array
Abstract
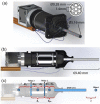
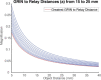
Optical endoscopy has emerged as an indispensable clinical tool for modern minimally invasive surgery. Most systems primarily capture a 2D projection of the 3D surgical field. Currently available 3D endoscopes can restore stereoscopic vision directly by projecting laterally shifted views of the operating field to each eye through 3D glasses. These tools provide surgeons with informative 3D visualizations, but they do not enable quantitative volumetric rendering of tissue. Therefore, advanced tools are desired to quantify tissue tomography for high precision microsurgery or medical robotics. Light-field imaging suggests itself as a promising solution to the challenge. The approach can capture both the spatial and angular information of optical signals, permitting the computational synthesis of the 3D volume of an object. In this work, we present GRIN lens array microendoscopy (GLAM), a single-shot, full-color, and quantitative 3D microendoscopy system. GLAM contains integrated fiber optics for illumination and a GRIN lens array to capture the reflected light field. The system exhibits a 3D resolution of ∼100 µm over an imaging depth of ∼22 mm and field of view up to 1 cm2. GLAM maintains a small form factor consistent with the clinically desirable design, making the system readily translatable to a clinical prototype.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest to this article.
Figures













References
-
- Sinha R., Sundaram M., Raje S., Rao G., Sinha M., Sinha R., “3D laparoscopy: technique and initial experience in 451 cases,” Gynecol Surg 10(2), 123–128 (2013). 10.1007/s10397-013-0782-8 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
