Optical phase modulation by natural eye movements: application to time-domain FF-OCT image retrieval
- PMID: 35284184
- PMCID: PMC8884228
- DOI: 10.1364/BOE.445393
Optical phase modulation by natural eye movements: application to time-domain FF-OCT image retrieval
Abstract
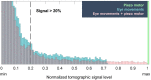
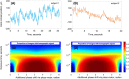
Eye movements are commonly seen as an obstacle to high-resolution ophthalmic imaging. In this context we study the natural axial movements of the in vivo human eye and show that they can be used to modulate the optical phase and retrieve tomographic images via time-domain full-field optical coherence tomography (TD-FF-OCT). This approach opens a path to a simplified ophthalmic TD-FF-OCT device, operating without the usual piezo motor-camera synchronization. The device demonstrates in vivo human corneal images under the different image retrieval schemes (2-phase and 4-phase) and different exposure times (3.5 ms, 10 ms, 20 ms). Data on eye movements, acquired with a spectral-domain OCT with axial eye tracking (180 B-scans/s), are used to study the influence of ocular motion on the probability of capturing high-signal tomographic images without phase washout. The optimal combinations of camera acquisition speed and amplitude of piezo modulation are proposed and discussed.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
VM: (P), PX: (P), MF: (P), ACB: (P).
Figures












References
-
- Drexler Wolfgang, Fujimoto James G., Optical Coherence Tomography: Technology and Applications , 2 (Springer International Publishing, 2015).
LinkOut - more resources
Full Text Sources
