COVID-19 vaccine acceptance and its associated factors in Ethiopia: A systematic review and meta-analysis
- PMID: 35284688
- PMCID: PMC8898789
- DOI: 10.1016/j.cegh.2022.101001
COVID-19 vaccine acceptance and its associated factors in Ethiopia: A systematic review and meta-analysis
Abstract
Background: COVID-19 vaccination is considered as an effective intervention for controlling the burden of the pandemic. However, vaccine hesitation is increasing and hindering efforts targeting to reduce the burden of the COVID-19 disease. Hence, determining COVID-19 vaccine acceptance and identifying determinants that would hinder people to vaccinate against COVID-19 is crucial to effectively improve COVID-19 vaccine uptake. In Ethiopia, the pooled proportion of COVID-19 vaccine acceptance and its determinants is not well known. Thus, the aim of this study is to estimate the pooled proportion of COVID-19 vaccine acceptance and its determinants in Ethiopia.
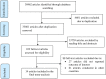
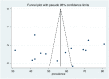
Methods: A systematic search of articles was conducted from PubMed, Scopus, Web of Science, MEDLINE, CINAHL, Science Direct and Cochrane Library. Data were extracted using a data extraction tool which was adapted from the Joanna Briggs Institute. The quality of each included primary studies was evaluated using the Newcastle-Ottawa scale tool. Data analysis was performed using STATA 14. Heterogeneity in studies was assessed using Cochrane Q and I2 test. Publication bias was assessed using visual inspection of funnel plots and Egger's test. A random effects model was applied to determine the pooled estimates if heterogeneity was exhibited; otherwise, a fixed-effects model was used.
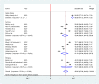
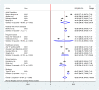
Results: A total of 14 studies involving 6373 participants were included for the final analysis. The pooled proportion of COVID-19 vaccine acceptance in Ethiopia was 56.02% (95% CI: 47.84, 64.20). The likelihood of COVID-19 vaccine acceptance was higher among participants who had history of chronic disease (AOR = 1.33, 95% CI: 1.09, 2.97), good knowledge (AOR = 2.13, 95% CI: 1.59, 4.97), positive attitude (AOR = 2.23, 95% CI: 1.21, 4.66), good COVID-19 preventive practice (AOR = 1.97, 95% CI: 1.82, 2.12), and high perceived seriousness of COVID-19 (AOR = 3.21, 95% CI: 2.32, 5.98).
Conclusion: More than half participants were willing to accept COVID-19 vaccine. Thus, awareness creation battles about the efficacy and safety of the COVID-19 vaccine should be provided to the community. Besides, policy-makers, health planners and other stakeholders should encourage COVID-19 vaccine uptake behaviors by providing trusted information.Systematic review and meta-analysis registration: PROSPERO CRD42021264708.
Keywords: AOR, Adjusted odd ratio; Acceptance; CI, Confidence intervals; COVID-19; COVID-19, Coronavirus disease 2019; Ethiopia; PRISMA, Preferred Reporting Items for Systematic review and Meta-analysis; Risk factors; SMP, self-medication practice; Vaccine; WHO, World Health Organization.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures


Similar articles
-
Determinants of COVID-19 vaccine acceptance in Ethiopia: A systematic review and meta-analysis.PLoS One. 2022 Jun 3;17(6):e0269273. doi: 10.1371/journal.pone.0269273. eCollection 2022. PLoS One. 2022. PMID: 35657814 Free PMC article.
-
Breast self-examination practice and associated factors among female healthcare workers in Ethiopia: A systematic review and meta-analysis.PLoS One. 2020 Nov 10;15(11):e0241961. doi: 10.1371/journal.pone.0241961. eCollection 2020. PLoS One. 2020. PMID: 33170880 Free PMC article.
-
Prevalence and associated factors of sexual violence experienced by housemaids in Ethiopia: a systematic review and meta-analysis.Reprod Health. 2022 Jul 19;19(1):162. doi: 10.1186/s12978-022-01470-2. Reprod Health. 2022. PMID: 35854381 Free PMC article.
-
COVID-19 preventive practice and associated factors in Ethiopia: A systematic review and meta-analysis.Public Health Pract (Oxf). 2022 Dec;4:100329. doi: 10.1016/j.puhip.2022.100329. Epub 2022 Oct 15. Public Health Pract (Oxf). 2022. PMID: 36267492 Free PMC article. Review.
-
COVID-19 vaccine acceptance and predictors among pregnant women in Ethiopia: Systematic Review and Meta-Analysis.Public Health Pract (Oxf). 2023 Jun;5:100386. doi: 10.1016/j.puhip.2023.100386. Epub 2023 Apr 23. Public Health Pract (Oxf). 2023. PMID: 37122635 Free PMC article. Review.
Cited by
-
COVID-19 vaccine hesitancy in Ethiopia: a latent class analysis.BMC Public Health. 2024 Oct 21;24(1):2894. doi: 10.1186/s12889-024-20359-2. BMC Public Health. 2024. PMID: 39434006 Free PMC article.
-
COVID-19 vaccine acceptance and associated factors in 13 African countries: A systematic review and meta-analysis.Front Public Health. 2023 Jan 24;10:1001423. doi: 10.3389/fpubh.2022.1001423. eCollection 2022. Front Public Health. 2023. PMID: 36761336 Free PMC article.
-
COVID-19 Vaccine Acceptance and Associated Factors Among College Students in Dessie City, Northeastern Ethiopia.J Multidiscip Healthc. 2022 Aug 13;15:1735-1746. doi: 10.2147/JMDH.S381151. eCollection 2022. J Multidiscip Healthc. 2022. PMID: 35990405 Free PMC article.
-
COVID-19 Vaccine Uptake and Socioeconomic Disparities in Tanzania: A Population-Based Cross-Sectional Study Amid High Hesitancy.Health Sci Rep. 2025 Jul 11;8(7):e71044. doi: 10.1002/hsr2.71044. eCollection 2025 Jul. Health Sci Rep. 2025. PMID: 40657298 Free PMC article.
-
COVID-19 Vaccine Coverage and Potential Drivers of Vaccine Uptake among Healthcare Workers in SOMALIA: A Cross-Sectional Study.Vaccines (Basel). 2022 Jul 13;10(7):1116. doi: 10.3390/vaccines10071116. Vaccines (Basel). 2022. PMID: 35891280 Free PMC article.
References
-
- Sage W. 2020. WHO SAGE Values Framework for the Allocation and Prioritization of COVID-19 Vaccination.
-
- Betsch C., Böhm R., Korn L., Holtmann C. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1–6.
Publication types
LinkOut - more resources
Full Text Sources
