Towards understanding transfluthrin efficacy in a pyrethroid-resistant strain of the malaria vector Anopheles funestus with special reference to cytochrome P450-mediated detoxification
- PMID: 35284893
- PMCID: PMC8906121
- DOI: 10.1016/j.crpvbd.2021.100041
Towards understanding transfluthrin efficacy in a pyrethroid-resistant strain of the malaria vector Anopheles funestus with special reference to cytochrome P450-mediated detoxification
Abstract
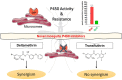
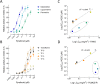
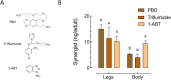
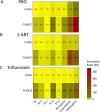
Malaria vector control interventions rely heavily on the application of insecticides against anopheline mosquitoes, in particular the fast-acting pyrethroids that target insect voltage-gated sodium channels (VGSC). Frequent applications of pyrethroids have resulted in resistance development in the major malaria vectors including Anopheles funestus, where resistance is primarily metabolic and driven by the overexpression of microsomal cytochrome P450 monooxygenases (P450s). Here we examined the pattern of cross-resistance of the pyrethroid-resistant An. funestus strain FUMOZ-R towards transfluthrin and multi-halogenated benzyl derivatives, permethrin, cypermethrin and deltamethrin in comparison to the susceptible reference strain FANG. Transfluthrin and two multi-fluorinated derivatives exhibited micromolar potency - comparable to permethrin - to functionally expressed dipteran VGSC in a cell-based cation influx assay. The activity of transfluthrin and its derivatives on VGSC was strongly correlated with their contact efficacy against strain FUMOZ-R, although no such correlation was obtained for the other pyrethroids due to their rapid detoxification by the resistant strain. The low resistance levels for transfluthrin and derivatives in strain FUMOZ-R were only weakly synergized by known P450 inhibitors such as piperonyl butoxide (PBO), triflumizole and 1-aminobenzotriazole (1-ABT). In contrast, deltamethrin toxicity in FUMOZ-R was synergized > 100-fold by all three P450 inhibitors. The biochemical profiling of a range of fluorescent resorufin and coumarin compounds against FANG and FUMOZ-R microsomes identified 7-benzyloxymethoxy-4-trifluoromethylcoumarin (BOMFC) as a highly sensitive probe substrate for P450 activity. BOMFC was used to develop a fluorescence-based high-throughput screening assay to measure the P450 inhibitory action of potential synergists. Azole fungicides prochloraz and triflumizole were identified as extremely potent nanomolar inhibitors of microsomal P450s, strongly synergizing deltamethrin toxicity in An. funestus. Overall, the present study contributed to the understanding of transfluthrin efficacy at the molecular and organismal level and identified azole compounds with potential to synergize pyrethroid efficacy in malaria vectors.
Keywords: Anopheles; Azole fungicides; Deltamethrin; FUMOZ-R; Malaria; P450; Pyrethroid resistance; Sodium channel; Synergists.
© 2021 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Andreas Brockmann, Kai-Uwe Brüggen, Ralf Nauen, Sebastian Horstmann and Ulrich Ebbinghaus-Kintscher are employed by Bayer AG, a manufacturer of pesticides. Melanie Nolden is a PhD student affiliated with the LSTM and funded by the Innovative Vector Control Consortium (IVCC) and 10.13039/100004326Bayer AG. Mark Paine declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Resilience of transfluthrin to oxidative attack by duplicated CYP6P9 variants known to confer pyrethroid resistance in the major malaria mosquito Anopheles funestus.Pestic Biochem Physiol. 2023 Apr;191:105356. doi: 10.1016/j.pestbp.2023.105356. Epub 2023 Feb 2. Pestic Biochem Physiol. 2023. PMID: 36963931
-
Biochemical profiling of functionally expressed CYP6P9 variants of the malaria vector Anopheles funestus with special reference to cytochrome b5 and its role in pyrethroid and coumarin substrate metabolism.Pestic Biochem Physiol. 2022 Mar;182:105051. doi: 10.1016/j.pestbp.2022.105051. Epub 2022 Feb 4. Pestic Biochem Physiol. 2022. PMID: 35249659
-
Contact Bioassays with Phenoxybenzyl and Tetrafluorobenzyl Pyrethroids against Target-Site and Metabolic Resistant Mosquitoes.PLoS One. 2016 Mar 1;11(3):e0149738. doi: 10.1371/journal.pone.0149738. eCollection 2016. PLoS One. 2016. PMID: 26930058 Free PMC article.
-
Sequential phase I metabolism of pyrethroids by duplicated CYP6P9 variants results in the loss of the terminal benzene moiety and determines resistance in the malaria mosquito Anopheles funestus.Insect Biochem Mol Biol. 2022 Sep;148:103813. doi: 10.1016/j.ibmb.2022.103813. Epub 2022 Jul 21. Insect Biochem Mol Biol. 2022. PMID: 35870762
-
RNAseq-based gene expression profiling of the Anopheles funestus pyrethroid-resistant strain FUMOZ highlights the predominant role of the duplicated CYP6P9a/b cytochrome P450s.G3 (Bethesda). 2022 Jan 4;12(1):jkab352. doi: 10.1093/g3journal/jkab352. G3 (Bethesda). 2022. PMID: 34718535 Free PMC article.
Cited by
-
Molecular markers of reduced behavioral sensitivity to transfluthrin in Anopheles gambiae s.s. from Western Kenya.BMC Genomics. 2025 Jun 5;26(1):565. doi: 10.1186/s12864-025-11755-y. BMC Genomics. 2025. PMID: 40474067 Free PMC article.
-
A conserved hymenopteran-specific family of cytochrome P450s protects bee pollinators from toxic nectar alkaloids.Sci Adv. 2023 Apr 14;9(15):eadg0885. doi: 10.1126/sciadv.adg0885. Epub 2023 Apr 12. Sci Adv. 2023. PMID: 37043574 Free PMC article.
-
Spatial repellents: The current roadmap to global recommendation of spatial repellents for public health use.Curr Res Parasitol Vector Borne Dis. 2022 Dec 9;3:100107. doi: 10.1016/j.crpvbd.2022.100107. eCollection 2023. Curr Res Parasitol Vector Borne Dis. 2022. PMID: 36590345 Free PMC article.
-
Semi-field evaluation of a volatile transfluthrin-based intervention reveals efficacy as a spatial repellent and evidence of other modes of action.PLoS One. 2023 May 11;18(5):e0285501. doi: 10.1371/journal.pone.0285501. eCollection 2023. PLoS One. 2023. PMID: 37167335 Free PMC article.
-
Phylogenomic and functional characterization of an evolutionary conserved cytochrome P450-based insecticide detoxification mechanism in bees.Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2205850119. doi: 10.1073/pnas.2205850119. Epub 2022 Jun 21. Proc Natl Acad Sci U S A. 2022. PMID: 35733268 Free PMC article.
References
-
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267.
-
- Amenya D.A., Naguran R., Lo T.-C.M., Ranson H., Spillings B.L., Wood O.R., et al. Over expression of a cytochrome P450 (CYP6P9) in a major African malaria vector. Insect Mol. Biol. 2008;17:19–25. - PubMed
-
- Balabanidou V., Grigoraki L., Vontas J. Insect cuticle: a critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018;27:68–74. - PubMed
-
- Behrenz W., Elbert A. Cyfluthrin and fenfluthrin, two new pyrethroidsfor the control of hygiene pests. Anzeiger für Schädlingskunde, Pflanzenschutz. Umweltschutz. 1985;58:30–35.
LinkOut - more resources
Full Text Sources

