Hematopoietic responses to SARS-CoV-2 infection
- PMID: 35284964
- PMCID: PMC8918078
- DOI: 10.1007/s00018-022-04220-6
Hematopoietic responses to SARS-CoV-2 infection
Abstract
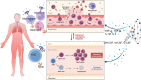
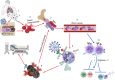
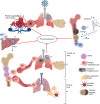
Under physiological conditions, hematopoietic stem and progenitor cells (HSPCs) in the bone marrow niches are responsible for the highly regulated and interconnected hematopoiesis process. At the same time, they must recognize potential threats and respond promptly to protect the host. A wide spectrum of microbial agents/products and the consequences of infection-induced mediators (e.g. cytokines, chemokines, and growth factors) can have prominent impact on HSPCs. While COVID-19 starts as a respiratory tract infection, it is considered a systemic disease which profoundly alters the hematopoietic system. Lymphopenia, neutrophilia, thrombocytopenia, and stress erythropoiesis are the hallmark of SARS-CoV-2 infection. Moreover, thrombocytopenia and blood hypercoagulability are common among COVID-19 patients with severe disease. Notably, the invasion of erythroid precursors and progenitors by SARS-CoV-2 is a cardinal feature of COVID-19 disease which may in part explain the mechanism underlying hypoxia. These pieces of evidence support the notion of skewed steady-state hematopoiesis to stress hematopoiesis following SARS-CoV-2 infection. The functional consequences of these alterations depend on the magnitude of the effect, which launches a unique hematopoietic response that is associated with increased myeloid at the expense of decreased lymphoid cells. This article reviews some of the key pathways including the infectious and inflammatory processes that control hematopoiesis, followed by a comprehensive review that summarizes the latest evidence and discusses how SARS-CoV-2 infection impacts hematopoiesis.
Keywords: COVID-19; Cytokine storm; Erythropoiesis; Hematopoietic stem and progenitors; Stress hematopoiesis.
© 2022. The Author(s).
Conflict of interest statement
The author has no relevant financial or non-financial interests to disclose.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

