Population Pharmacokinetics of Moxifloxacin in Children
- PMID: 35284983
- PMCID: PMC9768852
- DOI: 10.1007/s40272-022-00493-3
Population Pharmacokinetics of Moxifloxacin in Children
Abstract
Background/objective: Moxifloxacin is a fluoroquinolone that is commonly used in adults, but not children. Certain clinical situations compel pediatric clinicians to use moxifloxacin, despite its potential for toxicity and limited pharmacokinetics (PK) data. Our objective was to further characterize the pharmacokinetics of moxifloxacin in children.
Methods: We performed an opportunistic, open-label population PK study of moxifloxacin in children < 18 years of age who received moxifloxacin as part of standard care. A set of structural PK models and residual error models were explored using nonlinear mixed-effects modeling. Covariates with known biological relationships were investigated for their influence on PK parameters.
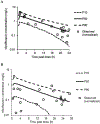
Results: We obtained 43 moxifloxacin concentrations from 14 participants who received moxifloxacin intravenously (n = 8) or orally (n = 6). The dose of moxifloxacin was 10 mg/kg daily in participants ≤ 40 kg and 400 mg daily in participants > 40 kg. The population mean clearance and mean volume of distribution were 18.2 L/h and 167 L, respectively. The oral absorption was described by a first-order process. The estimated extent of oral bioavailability was highly variable (range 20-91%). Total body weight was identified as a covariate on clearance and volume of distribution, and substantially reduced the random unexplained inter-individual variability for both parameters. No participants experienced suspected serious adverse reactions related to moxifloxacin.
Conclusion: These data add to the existing literature to support use of moxifloxacin in children in certain situations; however, further prospective studies on the safety and efficacy of moxifloxacin are needed.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Figures


References
-
- DAILYMED. MOXIFLOXACIN HYDROCHLORIDE - moxifloxacin hydrochloride tablet (drug label), Mylan Pharmaceuticals Inc. Revised August 2015. DAILYMED web site. https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid.... Accessed May 7, 2021.
-
- Stass H, Lettieri J, Vanevski KM, Willmann S, James LP, Sullivan JE, et al. Pharmacokinetics, safety, and tolerability of single-dose intravenous moxifloxacin in pediatric patients: dose optimization in a phase 1 study. J Clin Pharmacol. 2019;59(5):654–67. doi: 10.1002/jcph.1358. - DOI - PMC - PubMed
-
- Willmann S, Frei M, Sutter G, Coboeken K, Wendl T, Eissing T, et al. Application of physiologically-based and population pharmacokinetic modeling for dose finding and confirmation during the pediatric development of moxifloxacin. CPT Pharmacometrics Syst Pharmacol. 2019;8(9):654–63. doi: 10.1002/psp4.12446. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- FY2016A1B1C1D1.0026/this project was supported by the division of microbiology and infectious diseases (dmid), national institute of allergy and infectious diseases (niaid) of nih through the vaccine and treatment evaluation units (vteu), and the us department of health and h
- Duke University HHSN272201300017I/this project was supported by the division of microbiology and infectious diseases (dmid), national institute of allergy and infectious diseases (niaid) of nih through the vaccine and treatment evaluation units (vteu), and the us department of health and h
- Emmes HHSN272201500002C/this project was supported by the division of microbiology and infectious diseases (dmid), national institute of allergy and infectious diseases (niaid) of nih through the vaccine and treatment evaluation units (vteu), and the us department of health and h
- HHSN272201300017C/AI/NIAID NIH HHS/United States
- HHSN272201300017I/AI/NIAID NIH HHS/United States
- HHSN27200009/this project was supported by the division of microbiology and infectious diseases (dmid), national institute of allergy and infectious diseases (niaid) of nih through the vaccine and treatment evaluation units (vteu), and the us department of health and h
- HHSN272201500002C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

