Priority Effects in the Apple Flower Determine If the Siderophore Desferrioxamine Is a Virulence Factor for Erwinia amylovora CFBP1430
- PMID: 35285239
- PMCID: PMC9004392
- DOI: 10.1128/aem.02433-21
Priority Effects in the Apple Flower Determine If the Siderophore Desferrioxamine Is a Virulence Factor for Erwinia amylovora CFBP1430
Abstract
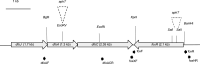
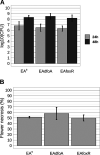
Iron is crucial for bacterial growth and virulence. Under iron-deficiency bacteria produce siderophores, iron chelators that facilitate the iron uptake into the cell via specific receptors. Erwinia amylovora, the causative agent of fire blight, produces hydroxamate-type desferrioxamine siderophores (DFO). The presented study reassesses the impact of DFO as a virulence factor of E. amylovora during its epiphytic phase on the apple flower. When inoculated in semisterile Golden Delicious flowers no difference in replication and induction of calyx necrosis could be observed between E. amylovora CFBP1430 siderophore synthesis (DfoA) or uptake (FoxR receptor) mutants and the parental strain. In addition, mutant strains only weakly induced a foxR promoter-gfpmut2 reporter construct in the flowers. When analyzing the replication of the receptor mutant in apple flowers harboring an established microbiome, either naturally, in case of orchard flowers, or by pre-inoculation of semisterile greenhouse flowers, it became evident that the mutant strain had a significantly reduced replication compared to the parental strain. The results suggest that apple flowers per se are not an iron-limiting environment for E. amylovora and that DFO is an important competition factor for the pathogen in precolonized flowers. IMPORTANCE Desferrioxamine is a siderophore produced by the fire blight pathogen E. amylovora under iron-limited conditions. In the present study, no or only weak induction of an iron-regulated promoter-GFP reporter was observed on semisterile apple flowers, and siderophore synthesis or uptake (receptor) mutants exhibited colonization of the flower and necrosis induction at parental levels. Reduced replication of the receptor mutant was observed when the flowers were precolonized by microorganisms. The results indicate that apple flowers are an iron-limited environment for E. amylovora only if precolonization with microorganisms leads to iron competition. This is an important insight for the timing of biocontrol treatments.
Keywords: Erwinia amylovora; apple flowers; desferrioxamine; iron deficiency; replication; secondary colonization; siderophore mutants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

