Lymphoma cell-derived extracellular vesicles inhibit autophagy and apoptosis to promote lymphoma cell growth via the microRNA-106a/Beclin1 axis
- PMID: 35285412
- PMCID: PMC9132475
- DOI: 10.1080/15384101.2022.2047335
Lymphoma cell-derived extracellular vesicles inhibit autophagy and apoptosis to promote lymphoma cell growth via the microRNA-106a/Beclin1 axis
Abstract
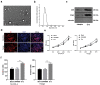
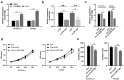
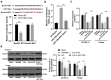
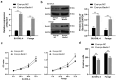
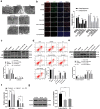
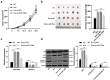
Lymphoma is a common malignant tumor globally. Tumor-derived extracellular vesicles (Evs) participate in genetic information exchange between tumor cells. We investigated the role and mechanism of human Burkitt lymphoma cells Raji-derived Evs (Raji-Evs) in lymphoma cells. Effects of Evs on lymphoma cell proliferation, invasion, autophagy, and apoptosis were assessed using Cell Counting Kit-8 method, Transwell assay, laser confocal microscopy, Western blotting, and flow cytometry. microRNA (miR)-106a expression in lymphoma cells was determined using reverse transcription-quantitative polymerase chain reaction and then downregulated in Raji cells and then Evs were isolated (Evs-in-miR-106a) to evaluate its role in lymphoma cell growth. The binding relationship between miR-106a and Beclin1 was verified using RNA pull-down and dual-luciferase assays. Beclin1 was overexpressed in SU-DHL-4 and Farage cells and SU-DHL-4 cell autophagy and apoptosis were detected. The levels of miR-106a and Beclin1 in SU-DHL-4 cells were detected after adding autophagy inhibitors. The tumorigenicity assay in nude mice was performed to validate the effects of Raji-Evs in vivo. Raji-Evs promoted lymphoma cell proliferation and invasion and increased miR-106a. miR-106a knockdown reversed Evs-promoted lymphoma cell proliferation and invasion. miR-106a carried by Raji-Evs targeted Beclin1 expression. Beclin1 overexpression or miR-106a inhibitor reversed the effects of Evs on lymphoma cell autophagy and apoptosis. Autophagy inhibitors elevated miR-106a expression and lowered Beclin1 expression. Raji-Evs-carried miR-106a inhibited Beclin1-dependent autophagy and apoptosis in lymphoma cells, which were further verified in vivo, together with promoted tumor growth. We proved that Raji-Evs inhibited lymphoma cell autophagy and apoptosis and promoted cell growth via the miR-106a/Beclin1 axis.
Keywords: Beclin1; Lymphoma cells; apoptosis; autophagy; extracellular vesicles; microRNA-106a.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
miR-106a-5p carried by tumor-derived extracellular vesicles promotes the invasion and metastasis of ovarian cancer by targeting KLF6.Clin Exp Metastasis. 2022 Aug;39(4):603-621. doi: 10.1007/s10585-022-10165-8. Epub 2022 Apr 21. Clin Exp Metastasis. 2022. PMID: 35449340
-
MicroRNA-185 inhibits cell proliferation while promoting apoptosis and autophagy through negative regulation of TGF-β1/mTOR axis and HOXC6 in nasopharyngeal carcinoma.Cancer Biomark. 2018;23(1):107-123. doi: 10.3233/CBM-181459. Cancer Biomark. 2018. Retraction in: Cancer Biomark. 2022;34(4):695. doi: 10.3233/CBM-220951. PMID: 29991129 Retracted.
-
Upregulation of p72 Enhances Malignant Migration and Invasion of Glioma Cells by Repressing Beclin1 Expression.Biochemistry (Mosc). 2016 Jun;81(6):574-82. doi: 10.1134/S0006297916060031. Biochemistry (Mosc). 2016. PMID: 27301285
-
Extracellular Vesicles Derived from Human Bone Marrow Stem Cells Inhibit Acute Lymphoblastic Leukemia Cell Growth by Inhibiting MAPK Pathway via the miR-29b-3p/GDF15 Axis.Acta Haematol. 2023;146(6):505-517. doi: 10.1159/000527456. Epub 2022 Nov 3. Acta Haematol. 2023. PMID: 36327876
-
Loading MicroRNA-376c in Extracellular Vesicles Inhibits Properties of Non-Small Cell Lung Cancer Cells by Targeting YTHDF1.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820977525. doi: 10.1177/1533033820977525. Technol Cancer Res Treat. 2020. PMID: 33280517 Free PMC article.
Cited by
-
The Role of Extracellular Vesicles in the Pathogenesis of Hematological Malignancies: Interaction with Tumor Microenvironment; a Potential Biomarker and Targeted Therapy.Biomolecules. 2023 May 27;13(6):897. doi: 10.3390/biom13060897. Biomolecules. 2023. PMID: 37371477 Free PMC article. Review.
-
MiR-106a targets ATG7 to inhibit autophagy and angiogenesis after myocardial infarction.Animal Model Exp Med. 2024 Aug;7(4):408-418. doi: 10.1002/ame2.12418. Epub 2024 May 28. Animal Model Exp Med. 2024. PMID: 38807299 Free PMC article.
-
Anti-inflammation is an important way that Qingre-Huazhuo-Jiangsuan recipe treats acute gouty arthritis.Front Pharmacol. 2023 Oct 10;14:1268641. doi: 10.3389/fphar.2023.1268641. eCollection 2023. Front Pharmacol. 2023. PMID: 37881185 Free PMC article.
-
Menthol induces extracellular vesicle regulation of apoptosis via ATG3 and caspase-3 in acute leukemic cells.Heliyon. 2024 Jun 18;10(12):e33081. doi: 10.1016/j.heliyon.2024.e33081. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39021955 Free PMC article.
-
Deciphering pancreatic neuroendocrine tumors: Unveiling through circulating small extracellular vesicles.Heliyon. 2024 Apr 1;10(7):e29079. doi: 10.1016/j.heliyon.2024.e29079. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38596136 Free PMC article.
References
-
- Armitage JO, Gascoyne RD, Lunning MA, et al. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298–310. - PubMed
-
- Yoo KH, Lee H, Suh C, et al. Lymphoma epidemiology in Korea and the real clinical field including the consortium for improving survival of lymphoma (CISL) trial. Int J Hematol. 2018;107(4):395–404. - PubMed
-
- Lewis WD, Lilly S, Jones KL.. Lymphoma: diagnosis and treatment. Am Fam Physician. 2020;101(1):34–41. - PubMed
-
- Mugnaini EN, Ghosh N.. Lymphoma. Prim Care. 2016;43(4):661–675. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
