Multiple Respiratory Syncytial Virus (RSV) Strains Infecting HEp-2 and A549 Cells Reveal Cell Line-Dependent Differences in Resistance to RSV Infection
- PMID: 35285685
- PMCID: PMC9006923
- DOI: 10.1128/jvi.01904-21
Multiple Respiratory Syncytial Virus (RSV) Strains Infecting HEp-2 and A549 Cells Reveal Cell Line-Dependent Differences in Resistance to RSV Infection
Abstract
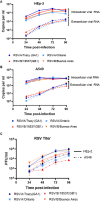
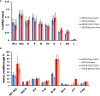
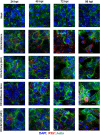
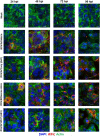
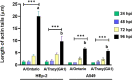
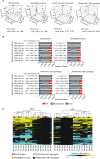
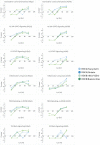
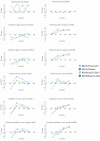
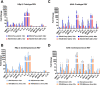
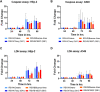
Respiratory syncytial virus (RSV) is a leading cause of pediatric acute respiratory infection worldwide. There are currently no approved vaccines or antivirals to combat RSV disease. A few transformed cell lines and two historic strains have been extensively used to study RSV. Here, we reported a thorough molecular and cell biological characterization of HEp-2 and A549 cells infected with one of four strains of RSV representing both major subgroups as well as historic and more contemporary genotypes (RSV/A/Tracy [GA1], RSV/A/Ontario [ON], RSV/B/18537 [GB1], and RSV/B/Buenos Aires [BA]) via measurements of viral replication kinetics and viral gene expression, immunofluorescence-based imaging of gross cellular morphology and cell-associated RSV, and measurements of host response, including transcriptional changes and levels of secreted cytokines and growth factors. IMPORTANCE Infection with the respiratory syncytial virus (RSV) early in life is essentially guaranteed and can lead to severe disease. Most RSV studies have involved either of two historic RSV/A strains infecting one of two cell lines, HEp-2 or A549 cells. However, RSV contains ample variation within two evolving subgroups (A and B), and HEp-2 and A549 cell lines are genetically distinct. Here, we measured viral action and host response in both HEp-2 and A549 cells infected with four RSV strains from both subgroups and representing both historic and more contemporary strains. We discovered a subgroup-dependent difference in viral gene expression and found A549 cells were more potently antiviral and more sensitive, albeit subtly, to viral variation. Our findings revealed important differences between RSV subgroups and two widely used cell lines and provided baseline data for experiments with model systems better representative of natural RSV infection.
Keywords: A549; HEp-2; RSV; cytokines; host gene expression; viral gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schobel SA, Stucker KM, Moore ML, Anderson LJ, Larkin EK, Shankar J, Bera J, Puri V, Shilts MH, Rosas-Salazar C, Halpin RA, Fedorova N, Shrivastava S, Stockwell TB, Peebles RS, Hartert TV, Das SR. 2016. Respiratory Syncytial Virus whole-genome sequencing identifies convergent evolution of sequence duplication in the C-terminus of the G gene. Sci Rep 6:26311. 10.1038/srep26311. - DOI - PMC - PubMed
-
- Hillyer P, Shepard R, Uehling M, Krenz M, Sheikh F, Thayer KR, Huang L, Yan L, Panda D, Luongo C, Buchholz UJ, Collins PL, Donnelly RP, Rabin RL. 2018. Differential responses by human respiratory epithelial cell lines to respiratory syncytial virus reflect distinct patterns of infection control. J Virol 92:e02202-17. 10.1128/JVI.02202-17. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

