Nitrogen Metabolism in Pseudomonas putida: Functional Analysis Using Random Barcode Transposon Sequencing
- PMID: 35285712
- PMCID: PMC9004399
- DOI: 10.1128/aem.02430-21
Nitrogen Metabolism in Pseudomonas putida: Functional Analysis Using Random Barcode Transposon Sequencing
Abstract
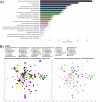
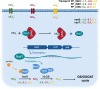
Pseudomonas putida KT2440 has long been studied for its diverse and robust metabolisms, yet many genes and proteins imparting these growth capacities remain uncharacterized. Using pooled mutant fitness assays, we identified genes and proteins involved in the assimilation of 52 different nitrogen containing compounds. To assay amino acid biosynthesis, 19 amino acid drop-out conditions were also tested. From these 71 conditions, significant fitness phenotypes were elicited in 672 different genes including 100 transcriptional regulators and 112 transport-related proteins. We divide these conditions into 6 classes, and propose assimilatory pathways for the compounds based on this wealth of genetic data. To complement these data, we characterize the substrate range of three promiscuous aminotransferases relevant to metabolic engineering efforts in vitro. Furthermore, we examine the specificity of five transcriptional regulators, explaining some fitness data results and exploring their potential to be developed into useful synthetic biology tools. In addition, we use manifold learning to create an interactive visualization tool for interpreting our BarSeq data, which will improve the accessibility and utility of this work to other researchers. IMPORTANCE Understanding the genetic basis of P. putida's diverse metabolism is imperative for us to reach its full potential as a host for metabolic engineering. Many target molecules of the bioeconomy and their precursors contain nitrogen. This study provides functional evidence linking hundreds of genes to their roles in the metabolism of nitrogenous compounds, and provides an interactive tool for visualizing these data. We further characterize several aminotransferases, lactamases, and regulators, which are of particular interest for metabolic engineering.
Keywords: BarSeq; Pseudomonas putida; RB-TnSeq; amino acid; aminotransferase; aminotransferases; biosensor; biosensors; lactam; metabolism; nitrate; nitrite; nitrogen; nitrogen metabolism; nucleotide; polyamine; t-SNE; transposon.
Conflict of interest statement
The authors declare a conflict of interest. J.D.K. has financial interests in Amyris, Ansa Biotechnologies, Apertor Pharma, Berkeley Yeast, Demetrix, Lygos, Napigen, ResVita Bio, and Zero Acre Farms.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

