Association of Low- and No-Calorie Sweetened Beverages as a Replacement for Sugar-Sweetened Beverages With Body Weight and Cardiometabolic Risk: A Systematic Review and Meta-analysis
- PMID: 35285920
- PMCID: PMC9907347
- DOI: 10.1001/jamanetworkopen.2022.2092
Association of Low- and No-Calorie Sweetened Beverages as a Replacement for Sugar-Sweetened Beverages With Body Weight and Cardiometabolic Risk: A Systematic Review and Meta-analysis
Abstract
Importance: There are concerns that low- and no-calorie sweetened beverages (LNCSBs) do not have established benefits, with major dietary guidelines recommending the use of water and not LNCSBs to replace sugar-sweetened beverages (SSBs). Whether LNCSB as a substitute can yield similar improvements in cardiometabolic risk factors vs water in their intended substitution for SSBs is unclear.
Objective: To assess the association of LNCSBs (using 3 prespecified substitutions of LNCSBs for SSBs, water for SSBs, and LNCSBs for water) with body weight and cardiometabolic risk factors in adults with and without diabetes.
Data sources: Medline, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception through December 26, 2021.
Study selection: Randomized clinical trials (RCTs) with at least 2 weeks of interventions comparing LNCSBs, SSBs, and/or water were included.
Data extraction and synthesis: Data were extracted and risk of bias was assessed by 2 independent reviewers. A network meta-analysis was performed with data expressed as mean difference (MD) or standardized mean difference (SMD) with 95% CIs. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) system was used to assess the certainty of the evidence.
Main outcomes and measures: The primary outcome was body weight. Secondary outcomes were other measures of adiposity, glycemic control, blood lipids, blood pressure, measures of nonalcoholic fatty liver disease, and uric acid.
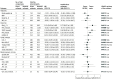
Results: A total of 17 RCTs with 24 trial comparisons were included, involving 1733 adults (mean [SD] age, 33.1 [6.6] years; 1341 women [77.4%]) with overweight or obesity who were at risk for or had diabetes. Overall, LNCSBs were a substitute for SSBs in 12 RCTs (n = 601 participants), water was a substitute for SSBs in 3 RCTs (n = 429), and LNCSBs were a substitute for water in 9 RCTs (n = 974). Substitution of LNCSBs for SSBs was associated with reduced body weight (MD, -1.06 kg; 95% CI, -1.71 to -0.41 kg), body mass index (MD, -0.32; 95% CI, -0.58 to -0.07), percentage of body fat (MD, -0.60%; 95% CI, -1.03% to -0.18%), and intrahepatocellular lipid (SMD, -0.42; 95% CI, -0.70 to -0.14). Substituting water for SSBs was not associated with any outcome. There was also no association found between substituting LNCSBs for water with any outcome except glycated hemoglobin A1c (MD, 0.21%; 95% CI, 0.02% to 0.40%) and systolic blood pressure (MD, -2.63 mm Hg; 95% CI, -4.71 to -0.55 mm Hg). The certainty of the evidence was moderate (substitution of LNCSBs for SSBs) and low (substitutions of water for SSBs and LNCSBs for water) for body weight and was generally moderate for all other outcomes across all substitutions.
Conclusions and relevance: This systematic review and meta-analysis found that using LNCSBs as an intended substitute for SSBs was associated with small improvements in body weight and cardiometabolic risk factors without evidence of harm and had a similar direction of benefit as water substitution. The evidence supports the use of LNCSBs as an alternative replacement strategy for SSBs over the moderate term in adults with overweight or obesity who are at risk for or have diabetes.
Conflict of interest statement
Figures



References
-
- Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576 - DOI - PMC - PubMed
-
- US Departments of Agriculture and Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. US Departments of Agriculture and Health and Human Services; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

