Fetal inflammatory response at the fetomaternal interface: A requirement for labor at term and preterm
- PMID: 35285967
- PMCID: PMC9188997
- DOI: 10.1111/imr.13075
Fetal inflammatory response at the fetomaternal interface: A requirement for labor at term and preterm
Abstract
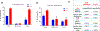
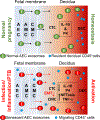
Human parturition at term and preterm is an inflammatory process synchronously executed by both fetomaternal tissues to transition them from a quiescent state t an active state of labor to ensure delivery. The initiators of the inflammatory signaling mechanism can be both maternal and fetal. The placental (fetal)-maternal immune and endocrine mediated homeostatic imbalances and inflammation are well reported. However, the fetal inflammatory response (FIR) theories initiated by the fetal membranes (amniochorion) at the choriodecidual interface are not well established. Although immune cell migration, activation, and production of proparturition cytokines to the fetal membranes are reported, cellular level events that can generate a unique set of inflammation are not well discussed. This review discusses derangements to fetal membrane cells (physiologically and pathologically at term and preterm, respectively) in response to both endogenous and exogenous factors to generate inflammatory signals. In addition, the mechanisms of inflammatory signal propagation (fetal signaling of parturition) and how these signals cause immune imbalances at the choriodecidual interface are discussed. In addition to maternal inflammation, this review projects FIR as an additional mediator of inflammatory overload required to promote parturition.
Keywords: EMT; aging; amniochorion; choriodecidua; exosomes; inflammation; premature rupture of the membranes; preterm birth; senescence; signaling.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
Dr. Menon's research work was partly supported by ILIAS Biologics, Daejeon, S Korea between 2019–2021.
Figures


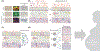
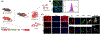

References
-
- Tal R, Taylor HS. Endocrinology of pregnancy. In: Feingold KR, Anawalt B, Boyce A, et al., Endotext. MDText.com, Inc.; 2000.
-
- Smith R Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol. 1998;39(1–2):215–220. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

