Checkpoint inhibitor immunotherapy during pregnancy for relapsed-refractory Hodgkin lymphoma
- PMID: 35285979
- PMCID: PMC9314600
- DOI: 10.1002/ajh.26527
Checkpoint inhibitor immunotherapy during pregnancy for relapsed-refractory Hodgkin lymphoma
Conflict of interest statement
Andrew M. Evens: Advisory Board (research related): Seattle Genetics, MorphoSys, Karyopharm, Novartis, Pharmacyclics, Abbvie, and Epizyme; all other authors: none.
Figures
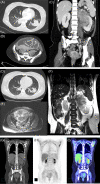
References
-
- Van Calsteren K, Heyns L, De Smet F, et al. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol. 2010;28:683‐689. - PubMed
-
- Dunleavy K, McLintock C. How I treat lymphoma in pregnancy. Blood. 2020;136:2118‐2124. - PubMed
-
- Evens AM, Advani R, Press OW, et al. Lymphoma occurring during pregnancy: antenatal therapy, complications, and maternal survival in a multicenter analysis. J Clin Oncol. 2013;31:4132‐4139. - PubMed
-
- Lishner M, Avivi I, Apperley JF, et al. Hematologic malignancies in pregnancy: management guidelines from an international consensus meeting. J Clin Oncol. 2016;34:501‐508. - PubMed
-
- Saliba AN, Xie Z, Higgins AS, et al. Immune‐related hematologic adverse events in the context of immune checkpoint inhibitor therapy. Am J Hematol. 2021;96:E362‐E367. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
