In vitro α-glucosidase inhibitory activity of Tamarix nilotica shoot extracts and fractions
- PMID: 35286313
- PMCID: PMC8920278
- DOI: 10.1371/journal.pone.0264969
In vitro α-glucosidase inhibitory activity of Tamarix nilotica shoot extracts and fractions
Abstract
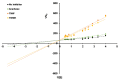
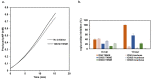
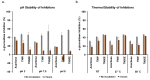
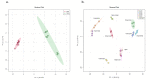
α-glucosidase inhibitors represent an important class of type 2 antidiabetic drugs and they act by lowering postprandial hyperglycemia. Today, only three synthetic inhibitors exist on the market, and there is a need for novel, natural and more efficient molecules exhibiting this activity. In this study, we investigated the ability of Tamarix nilotica ethanolic and aqueous shoot extracts, as well as methanolic fractions prepared from aqueous crude extracts to inhibit α-glucosidase. Both, 50% ethanol and aqueous extracts inhibited α-glucosidase in a concentration-dependent manner, with IC50 values of 12.5 μg/mL and 24.8 μg/mL, respectively. Importantly, α-glucosidase inhibitory activity observed in the T. nilotica crude extracts was considerably higher than pure acarbose (IC50 = 151.1 μg/mL), the most highly prescribed α-glucosidase inhibitor on the market. When T. nilotica crude extracts were fractionated using methanol, enhanced α-glucosidase inhibitory activity was observed in general, with the highest observed α-glucosidase inhibitory activity in the 30% methanol fraction (IC50 = 5.21 μg/mL). Kinetic studies further revealed a competitive reversible mechanism of inhibition by the plant extract. The phytochemical profiles of 50% ethanol extracts, aqueous extracts, and the methanolic fractions were investigated and compared using a metabolomics approach. Statistical analysis revealed significant differences in the contents of the crude extracts and fractions and potentially identified the molecules that were most responsible for these observed variations. Higher α-glucosidase inhibitory activity was associated with an enrichment of terpenoids, fatty acids, and flavonoids. Among the identified molecules, active compounds with known α-glucosidase inhibitory activity were detected, including unsaturated fatty acids, triterpenoids, and flavonoid glycosides. These results put forward T. nilotica as a therapeutic plant for type 2 diabetes and a source of α-glucosidase inhibitors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al.. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157: 107843. doi: 10.1016/j.diabres.2019.107843 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

