Testing the reliability and ecological implications of ramping rates in the measurement of Critical Thermal maximum
- PMID: 35286353
- PMCID: PMC8920270
- DOI: 10.1371/journal.pone.0265361
Testing the reliability and ecological implications of ramping rates in the measurement of Critical Thermal maximum
Abstract
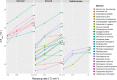
Critical Thermal maximum (CTmax) is often used to characterize the upper thermal limits of organisms and represents a key trait for evaluating the fitness of ectotherms. The lack of standardization in CTmax assays has, however, introduced methodological problems in its measurement, which can lead to questionable estimates of species' upper thermal limits. Focusing on ants, which are model organisms for research on thermal ecology, we aim to obtain a reliable ramping rate that will yield the most rigorous measures of CTmax for the most species. After identifying three commonly used ramping rates (i.e., 0.2, 0.5 and 1.0°C min-1) in the literature, we experimentally determine their effects on the CTmax values of 27 species measured using dynamic assays. Next, we use static assays to evaluate the accuracy of these values in function of the time of exposure. Finally, we use field observations of species' foraging activities across a wide range of ground temperatures to identify the most biologically relevant CTmax values and to develop a standardized method. Our results demonstrate that the use of a 1°C min-1 ramping rate in dynamic assays yields the most reliable CTmax values for comparing ant species' upper thermal limits, which are further validated in static assays and field observations. We further illustrate how methodological biases in physiological trait measurements can affect subsequent analyses and conclusions on community comparisons between strata and habitats, and the detection of phylogenetic signal (Pagel's λ and Bloomberg's K). Overall, our study presents a methodological framework for identifying a reliable and standardized ramping rate to measure CTmax in ants, which can be applied to other ectotherms. Particular attention should be given to CTmax values obtained with less suitable ramping rates, and the potential biases they may introduce to trait-based research on global warming and habitat conversion, as well as inferences about phylogenetic conservatism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Hoffmann AA, Chown SL, Clusella-Trullas S. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct Ecol. 2013;27(4):934–49. 10.1111/j.1365-2435.2012.02036.x. - DOI
-
- Lutterschmidt WI, Hutchison VH. The critical thermal maximum: history and critique. Can J Zool. 1997;75(10):1561–74. 10.1139/z97-783. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

