The human AP-endonuclease 1 (APE1) is a DNA G-quadruplex structure binding protein and regulates KRAS expression in pancreatic ductal adenocarcinoma cells
- PMID: 35286386
- PMCID: PMC8990529
- DOI: 10.1093/nar/gkac172
The human AP-endonuclease 1 (APE1) is a DNA G-quadruplex structure binding protein and regulates KRAS expression in pancreatic ductal adenocarcinoma cells
Abstract
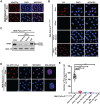
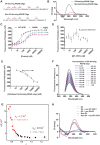
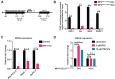
Pancreatic ductal adenocarcinoma (PDAC), one of the most aggressive types of cancer, is characterized by aberrant activity of oncogenic KRAS. A nuclease-hypersensitive GC-rich region in KRAS promoter can fold into a four-stranded DNA secondary structure called G-quadruplex (G4), known to regulate KRAS expression. However, the factors that regulate stable G4 formation in the genome and KRAS expression in PDAC are largely unknown. Here, we show that APE1 (apurinic/apyrimidinic endonuclease 1), a multifunctional DNA repair enzyme, is a G4-binding protein, and loss of APE1 abrogates the formation of stable G4 structures in cells. Recombinant APE1 binds to KRAS promoter G4 structure with high affinity and promotes G4 folding in vitro. Knockdown of APE1 reduces MAZ transcription factor loading onto the KRAS promoter, thus reducing KRAS expression in PDAC cells. Moreover, downregulation of APE1 sensitizes PDAC cells to chemotherapeutic drugs in vitro and in vivo. We also demonstrate that PDAC patients' tissue samples have elevated levels of both APE1 and G4 DNA. Our findings unravel a critical role of APE1 in regulating stable G4 formation and KRAS expression in PDAC and highlight G4 structures as genomic features with potential application as a novel prognostic marker and therapeutic target in PDAC.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Siegel R.L., Miller K.D., Jemal A.. Cancer statistics, 2020. CA Cancer J. Clin. 2020; 70:7–30. - PubMed
-
- Al Haddad A.H., Adrian T.E.. Challenges and future directions in therapeutics for pancreatic ductal adenocarcinoma. Expert Opin. Investig. Drugs. 2014; 23:1499–1515. - PubMed
-
- Hidalgo M., Cascinu S., Kleeff J., Labianca R., Lohr J.M., Neoptolemos J., Real F.X., Van Laethem J.L., Heinemann V.. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015; 15:8–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

