Efficacy of rituximab versus tacrolimus in difficult-to-treat steroid-sensitive nephrotic syndrome: an open-label pilot randomized controlled trial
- PMID: 35286456
- PMCID: PMC8919684
- DOI: 10.1007/s00467-022-05475-8
Efficacy of rituximab versus tacrolimus in difficult-to-treat steroid-sensitive nephrotic syndrome: an open-label pilot randomized controlled trial
Abstract
Background: Rituximab and tacrolimus are therapies reserved for patients with frequently relapsing or steroid-dependent nephrotic syndrome who have failed conventional steroid-sparing agents. Given their toxicities, demonstrating non-inferiority of rituximab to tacrolimus may enable choice between these medications.
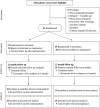
Methods: This investigator-initiated, single-center, open-label, pilot randomized controlled trial examined the non-inferiority of two doses of intravenous (IV) rituximab given one-week apart to oral therapy with tacrolimus (1:1 allocation), in maintaining sustained remission over 12 months follow-up, in patients with difficult-to-treat steroid-sensitive nephrotic syndrome, defined as frequently relapsing or steroid-dependent disease that had failed ≥ 2 steroid-sparing strategies. Secondary outcomes included frequency of relapses, proportion with frequent relapses, time to relapse and frequent relapses, and adverse events (CTRI/2018/11/016342).
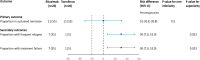
Results: Baseline characteristics were comparable for 41 patients randomized to receive rituximab (n = 21) or tacrolimus (n = 20). While 55% of patients in each limb were in sustained remission at 1 year, non-inferiority of rituximab to tacrolimus was not demonstrated (mean difference 0%; 95% CI - 30.8%, 30.8%; non-inferiority limit - 20%; P = 0.50). Frequent relapses were more common in patients administered rituximab compared to tacrolimus (risk difference 30%, 95% CI 7.0, 53.0, P = 0.023). Both groups showed similar reductions in relapse rates and prednisolone use. Common adverse events were infusion-related with rituximab and gastrointestinal symptoms with tacrolimus.
Conclusions: Therapy with rituximab was not shown to be non-inferior to 12-months treatment with tacrolimus in maintaining remission in patients with difficult-to-treat steroid-sensitive nephrotic syndrome. Frequent relapses were more common with rituximab. While effective, both agents require close monitoring for adverse events. A higher resolution version of the Graphical abstract is available as Supplementary information.
Keywords: Children; Frequently relapsing nephrotic syndrome; Rituximab; Steroid dependence; Tacrolimus.
© 2022. The Author(s), under exclusive licence to International Pediatric Nephrology Association.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

